
4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene synthesis
- Product Name:4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene
- CAS Number:161265-03-8
- Molecular formula:C39H32OP2
- Molecular Weight:578.62

19814-75-6

1079-66-9

161265-03-8
Example 2A Preparation of 9,9-dimethyl-4,5-bis(diphenylphosphino)xanthene (Xantphos): To a 5L round bottom flask was added methyl tert-butyl ether (MTBE, 2.5L), 9,9-dimethylxanthene (131.4 g, 0.60 mol) and N,N,N',N'-tetramethylethylenediamine (TMEDA, 155 g, 1.32 mol). After degassing the solution, sec-butyl lithium (s-BuLi, 1.11 L, 1.3 M in cyclohexane, 1.44 mol) was loaded into a dropping funnel, and then slowly added dropwise over a period of 60 min while controlling the reaction temperature at 6-10 °C. After the dropwise addition, the mixture was stirred at room temperature for 14 hours. Subsequently, diphenylphosphonium chloride (Ph2PCl) was slowly added through the dropping funnel while controlling the reaction temperature at 10-20°C to maintain a mild exothermic reaction. About 60% diphenylphosphonium chloride (175 mL, 0.93 mol) was added over 0.5 hr. The mixture was stirred for 10 minutes and then the remaining diphenylphosphonium chloride (120 mL, 0.63 mol) was added. The reaction mixture was continued to be stirred at room temperature for 5 hours before the reaction was quenched with methanol (MeOH, 9.9 mL, 0.24 mol). Filtration yielded a pale yellow solid product, which was washed sequentially with methyl tert-butyl ether (MTBE, 250 mL), methanol (2 x 250 mL), water (2 x 300 mL), methanol (2 x 250 mL) and methyl tert-butyl ether (250 mL), and dried to give an off-white solid product (304.2 g, 88% yield).
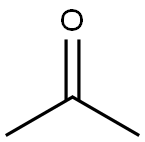
67-64-1
0 suppliers
$19.10/10ml
166330-10-5
323 suppliers
$9.00/1g

161265-03-8
472 suppliers
$6.00/1g
Yield:161265-03-8 95%
Reaction Conditions:
with trifluorormethanesulfonic acid in toluene at 200; for 16 h;Inert atmosphere;Temperature;
Steps:
1; 2; 3 Example 3
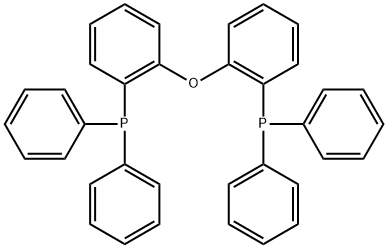
Under argon protection, bis(2-diphenylphosphinophenyl)ether (Compound 2) 538 g, toluene solvent was added to the reaction vessel.700 mL, 8.8 mL of trifluoromethanesulfonic acid, 150 mL of acetone, the reaction system was heated to 200 °C, and after 16 h of reaction, it was reduced toAt room temperature, after extraction, drying and recrystallization, the desired product 4,5-bisdiphenylphosphino-9,9-dimethyloxaxan (Compound 1) is obtained.549 g (yield 95%).
References:
CN109942631,2019,A Location in patent:Paragraph 0005; 0008-0009

19814-75-6
230 suppliers
$6.00/1g

1079-66-9
447 suppliers
$5.00/5g

161265-03-8
472 suppliers
$6.00/1g

19814-75-6
230 suppliers
$6.00/1g

161265-03-8
472 suppliers
$6.00/1g

808142-24-7
23 suppliers
inquiry

161265-03-8
472 suppliers
$6.00/1g