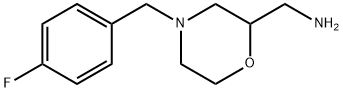
4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine synthesis
- Product Name:4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine
- CAS Number:112914-13-3
- Molecular formula:C12H17FN2O
- Molecular Weight:224.27
![1H-Isoindole-1,3(2H)-dione, 2-[[4-[(4-fluorophenyl)methyl]-2-morpholinyl]methyl]-](/CAS/20210305/GIF/636582-78-0.gif)
636582-78-0
0 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
155 suppliers
$5.00/250mg
Yield:112914-13-3 90%
Reaction Conditions:
with hydrazine in ethanol; for 3 h;Heating / reflux;
Steps:
7.D Example 7; Synthesis of 2-aminomethyl-4- (4-fluoro-benzyl) morphline (VII); D.)
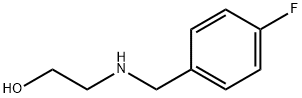
16.2 g (265 [MMOL)] of 2-amino-ethanol, 27.3 g (220 [MMOL)] of [4-FLUORO-BENZALDEHYDE] and 27.6 g of sodium-hydrogen-carbonate are refluxed in 260 ml of methanol with stirring for 4 hours and then 5.00 g (132 [MMOL)] of [SODIUM-BOROHYDRIDE] is added at 0-5 [C] for 2 hours. The reaction mixture is allowed to warm to room temperature, after the evolution of hydrogen is stopped filtered and evaporated under reduced pressure. The residue is solved in [DICHLOROMETHANE,] washed 2 x with saturated sodium chloride solution, dried over sodium sulphate, filtered and evaporated under reduced pressure to give 32.8 g (88%) of [2- (4-] fluoro-benzyl-amino)-ethanol as colourless, viscous oil. B) To 15.0 g (88.7 [MMOL)] of [2- (4-FLUORO-BENZYL-AMINO)-ETHANOL] obtained in the above (A) step 8.65 g (92.5 [MMOL)] of [EPICHLOROHYDRIN] is added, the mixture stirred 4.5 hours, 66 g of concentrated sulphuric acid added during 15 minutes and warmed at 150 [C] for 30 minutes. After cooling the sulphuric acid solution is poured into ice/water, treated with saturated sodium hydroxide solution and extracted with chloroform. The extract is washed with saturated sodium chloride solution, dried over sodium sulphate, filtered and evaporated under reduced pressure, to give 16.1 g (75%) of [2-CHLORO-METHYL-4- (4-FLUORO-BENZYL)-] morpholine as yellow, viscous oil. C) 12.3 g (50.5 [MMOL)] of [2-CHLORO-METHYL-4- (4-FLUORO-BENZYL)-MORPHOLINE] obtained in the above (B) step is solved in 195 ml of [ANHYDROUS N, N-DIMETHYL-FORMAMIDE] and 10.6 g (57.2 [MMOL)] of potassium-phthalimide added to the solution. The suspension is [REFLUXED] for 1.5 hours, then cooled and filtered. The solvent is evaporated under reduced pressure, the residue solved in chloroform and washed with water four times, dried over sodium sulphate, filtered and evaporated under reduced pressure. The residue is recrystallized from isopropanol to give 13.5 g (75%) of nearly white [2- [4- (4-FLUORO-BENZYL)-MORPHOLINE-2YL-METHYL]-ISOINDOLE-] 1.3-dion crystals. Mp: 150-151 [C.] D) 10.0 g (28.1 [MMOL)] of [2- [4- (4-FLUORO-BENZYL)-MORPHOLINE-2YL-METHYL]-ISOINDOLE-1. 3-DION] obtained in the above (C) step is suspended in the solution of 5.32 g (106 [MMOL)] of [HYDRAZINE-MONOHYDRATE] and 170 mi of ethanol and refluxed for 3 hours. The precipitation is removed by filtration and the ethanol solvent is evaporated under reduced pressure. The residue is solved in water and the solution extracted with [DICHLOROMETHANE.] The organic phase is washed 2 x with water, dried over sodium sulphate, filtered and evaporated under reduced pressure, to give 5.67 g (90%) of the title compound as yellow transparent oil.
References:
WO2003/106440,2003,A2 Location in patent:Page 10-11
5455-98-1
175 suppliers
$25.00/5g
22116-33-2
99 suppliers
$35.00/200μmol
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
155 suppliers
$5.00/250mg

112913-94-7
81 suppliers
$8.00/10g
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
155 suppliers
$5.00/250mg
![Acetamide, N-[[4-(phenylmethyl)-2-morpholinyl]methyl]-](/CAS/20210305/GIF/112913-96-9.gif)
112913-96-9
2 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
155 suppliers
$5.00/250mg

131322-23-1
0 suppliers
inquiry
![4-[(4-Fluorophenyl)methyl]-2-morpholinemethanamine](/CAS/GIF/112914-13-3.gif)
112914-13-3
155 suppliers
$5.00/250mg