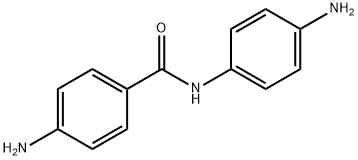
4,4'-Diaminobenzanilide synthesis
- Product Name:4,4'-Diaminobenzanilide
- CAS Number:785-30-8
- Molecular formula:C13H13N3O
- Molecular Weight:227.26
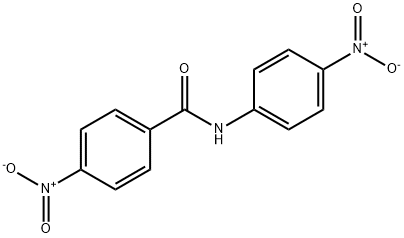
6333-15-9

785-30-8
1. 30 g of 4,4'-dinitrobenzanilide obtained in step 1, 150 mL of N,N'-dimethylformamide and 0.8 g of 3 wt% palladium-carbon catalyst were added to an autoclave. The hydrogenation reaction was carried out at a hydrogen pressure of 0.8±0.1 MPa and a temperature of 100±1 °C until the reaction was complete. Upon completion of the reaction, the reaction mixture was filtered and the solvent was evaporated under vacuum to obtain the crude 4,4'-diaminobenzanilide. 2. The crude 4,4'-diaminobenzanilide obtained in step 2 was added to an autoclave with 100 g of n-butanol and 0.96 g of activated carbon, and nitrogen was passed through for three times to ensure that a positive pressure was maintained in the autoclave. Subsequently, the temperature was raised to 135±1 °C and stirred for 20 min. The filtrate was filtered under nitrogen protection, the filtrate was cooled to 25±1 °C, filtered again and dried. Finally, 22.8 g of electronic grade 4,4'-diaminobenzanilide in the form of white crystalline powder was obtained in 96.1% yield and 99.7% purity (HPLC).

6333-15-9
3 suppliers
inquiry

785-30-8
335 suppliers
$5.00/1g
Yield:785-30-8 96.1%
Reaction Conditions:
Stage #1:4,4'-dinitrobenzanilide with palladium on activated charcoal;hydrogen in N,N-dimethyl-formamide at 10; under 6000.6 Torr;Autoclave;
Stage #2: in butan-1-ol at 25 - 135; for 0.333333 h;Autoclave;
Steps:
4 The preparation method of the electronic grade 4,4'-diaminobenzanilide of this embodiment has the following steps
2 To the autoclave, 30 g of 4,4'-dinitrobenzanilide obtained in step 1, 150 mL of N,N'-dimethylformamide, and 0.8 g of a 3 wt% palladium carbon catalyst were added. Into the hydrogen,The pressure-holding reaction was completed to completion at a pressure of 0.8 ± 0.1 MPa and a temperature of 100 ± 1 °C.After completion of the reaction, the mixture was filtered, and the solvent was evaporated to vacuo.The crude 4,4'-diaminobenzanilide was obtained.3 The crude 4,4'-diaminobenzanilide obtained in the step 2 was further added to the autoclave, 100 g of n-butanol and 0.96 g of activated carbon were added, and the nitrogen was replaced three times, and the nitrogen gas was discharged until the inside of the autoclave was maintained at a positive pressure.Then, the temperature was raised to 135±1° C., stirred for 20 min, filtered under nitrogen, and the filtrate was cooled to 25±1° C., filtered, and dried.22.8 g of an electronic grade 4,4'-diaminobenzanilide having a white crystalline powder was obtained in a yield of 96.1% and a purity of 99.7% (HPLC).
References:
Jiangsu Shang Laite Pharmaceutical And Chemical Materials Co., Ltd.;Hu Guoyi;Hu Jinping;Wu Jianhua;Zhang Peifeng;Xi Xiaojin CN108329224, 2018, A Location in patent:Paragraph 0013; 0015-0017; 0026; 0028-0030

122-04-3
37 suppliers
$15.00/1g

785-30-8
335 suppliers
$5.00/1g

100-01-6
66 suppliers
$11.19/25G

785-30-8
335 suppliers
$5.00/1g
62-23-7
732 suppliers
$14.00/25g

785-30-8
335 suppliers
$5.00/1g
