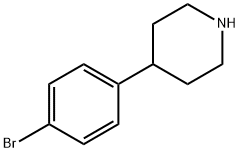
4-(4'-Bromophenyl)piperidine synthesis
- Product Name:4-(4'-Bromophenyl)piperidine
- CAS Number:80980-89-8
- Molecular formula:C11H14BrN
- Molecular Weight:240.14

91347-99-8

80980-89-8
General procedure for the synthesis of 4-(4-bromophenyl)piperidine from 4-(4-bromophenyl)-1,2,3,6-tetrahydropyridine: to a solution of 4-(4-bromophenyl)-1,2,3,6-tetrahydropyridine (0.90 g, 3.78 mmol) in anhydrous methanol (20 mL) and triethylamine (2 mL), the Rh/C catalyst (0.060 g, J. Bishop & Co. Platinum). The reaction mixture was placed in a hydrogen atmosphere (100 psi) and stirred at room temperature for 24 hours. Upon completion of the reaction, the mixture was filtered through a Celite?(Sigma Aldrich, St. Louis, MO) filter cake and the filtrate was concentrated to afford 4-(4-bromophenyl)piperidine as a white solid (0.91 g, 98% yield). The product was confirmed by 1H NMR (400 MHz, MeOD): δ 1.55-1.59 (2H, m), 1.61-1.70 (2H, m), 2.55-2.56 (1H, m), 2.64-2.70 (2H, m), 3.09-3.06 (2H, m), 7.13 (2H, J = 8.0 Hz, d), 7.31 ( 2H, J = 8.0 Hz, d).13C NMR (100 MHz, MeOD): δ 32.7, 41.4, 45.6, 119.4, 126.4, 128.2, 128.5, 131.2, 145.3. m/z of mass spectrum (ESI-MS): 241 [M+H]+.

91347-99-8
47 suppliers
$59.00/100 mg

80980-89-8
138 suppliers
$40.00/500mg
Yield:80980-89-8 98%
Reaction Conditions:
with rhodium contaminated with carbon;hydrogen;triethylamine in methanol at 20; under 5171.62 Torr; for 24 h;
Steps:
5 This example demonstrates the synthesis of the intermediate 4-(4- bromophenyl)piperidine (18) in an embodiment of the invention (Fig. 2).c
To a solution of 4-(4-bromophenyl)-1,2,3,6-tetrahydropyridine 17 (0.90 g, 3.78mmol) in dry MeOH (20 mL) and Et3N (2 ml) was added RhIC catalyst (0.060 g, J. Bishop & Co. Platinum). The resulting reaction mixture was stirred at room temperature in a hydrogen atmosphere (100 psi) for 24 h. The mixture was filtered through a cake of CELITETM (Sigma Aldrich, St. Louis, MO), and the filtrate was concentrated to give the title compound as a white solid (0.91 g. 98%). ‘H NMR (400 MHz, MeOD) 3; 1.55-159 (2H, m). 1.61-1.70 (2H, m), 2.55-2.56 (IH, m), 264-2.7C (2H. m), 3.09-3.06 (2H, m). 7.13 (2H. J = 8.0. d), 7.31 (2H, J = 8.0. d). ‘3CNMR(100 MHz, MeOD) 3: 32,7, 41.4, 45.6. 119.4. 126.4, 128,2, 128.5, 131.2, 145.3, m/z (ESI. MF1) 241.
References:
THE UNITED STATES OF AMERICA, AS REPRESENTED BY THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES;JACOBSON, Kenneth A.;JUNKER, Anna;ULIASSI, Elisa;KISELEV, Evgeny WO2017/53769, 2017, A1 Location in patent:Paragraph 0117; 0118
769944-78-7
100 suppliers
$19.00/250mg

80980-89-8
138 suppliers
$40.00/500mg

771-99-3
298 suppliers
$9.00/250mg

80980-89-8
138 suppliers
$40.00/500mg

188863-87-8
7 suppliers
inquiry

80980-89-8
138 suppliers
$40.00/500mg

3612-20-2
463 suppliers
$6.00/10g

80980-89-8
138 suppliers
$40.00/500mg