
4-(4-BROMOPHENYL)BUTANOIC ACID synthesis
- Product Name:4-(4-BROMOPHENYL)BUTANOIC ACID
- CAS Number:35656-89-4
- Molecular formula:C10H11BrO2
- Molecular Weight:243.1

6340-79-0

35656-89-4
General procedure for the synthesis of 4-(4-bromophenyl)butyric acid from 3-(4-bromobenzoyl)propionic acid: zinc powder (13.0 g, 200 mmol) was stirred with mercuric chloride (1.00 g, 4.80 mmol) in water (10 mL) and concentrated hydrochloric acid (0.6 mL) for 5 min. After decanting the liquid, toluene (20 mL), concentrated hydrochloric acid (20 mL) and water (8 mL) were added sequentially. 3-(4-Bromobenzoyl)propionic acid (2.55 g, 10.5 mmol) was added to the reaction system and heated at reflux at 100 °C for 24 h, during which concentrated hydrochloric acid (1 mL) was replenished every 6 hours. After completion of the reaction, it was cooled to room temperature, filtered, and the solvent was removed from the organic phase to give a clarified liquid, which precipitated as white crystals upon cooling. The product was purified by silica gel column chromatography (eluent: ethyl acetate/hexane, 1:3, v/v) to afford 4-(4-bromophenyl)butanoic acid (2.33 g, 9.63 mmol, 91.4% yield). Mass spectrometry (ESI-quadrupole) m/z: calculated values of C10H11BrO2[M-H]-: 241.99, 243.99; measured values: 244.0 (10%), 243.0 (98%), 242.0 (11%), 241.0 (100%) (negative ion mode). High performance liquid chromatography (HPLC) retention time: 27.7 min. 1H NMR (300 MHz, CDCl3) δ (ppm): 7.40 (2H, d, J = 8.4 Hz, ArH), 7.06 (2H, d, J = 8.4 Hz, ArH), 2.63 (2H, t, J = 7.8 Hz, CH2), 2.36 (2H, t, J = 7.2 Hz, CH2), 1.97 (2H, dt, J = 7.2, 7.8 Hz, CH2), OH was not observed.

6340-79-0
161 suppliers
$5.00/250mg

35656-89-4
128 suppliers
$25.00/1g
Yield:35656-89-4 91.4%
Reaction Conditions:
with hydrogenchloride;mercury(II) chloride;zinc in water;toluene at 100; for 24 h;
Steps:
1 4-(4-bromophenyl)butanoic acid 5
Zinc (13.0 g, 200 mmol) and mercury chloride (1 .00 g, 480 mmol) were stirred with water (10 mL) and concentrated HCI (0.6 mL) for five minutes. The liquid was decanted off and toluene (20 mL), concentrated HCI (20 mL) and water (8 mL) were added consecutively. 4-(4-bromophenyl)-4-oxobutanoic acid 4 (2.55 g, 10.5 mmol) was added and heated under reflux at 100°C for 24 h adding HCI (1 mL) every 6 h. The reaction was allowed to cool to room temperature, filtered and the solvent removed from the organic layer to give a clear liquid which gave white crystals upon cooling. These were purified with silica gel chromatography (ethyl acetate : hexane, 1 :3) to yield 4-(4- bromophenyl)butanoic acid 5 (2.33 g, 9.63 mmol, 91 .4%). MS (ESI-QUADRUPOLE) m/z: calc. for CioHn BrO2: 241 .99, 243.99; Found: 244.0 (10), 243.0 (98), 242.0 (1 1 ), 241 .0 (100) (negative ions). HPLC: tr = 27.7 min. 1 H NMR (300 MHz, CDCI3) (δ/ppm) 7.40 (2H, d, J = 8.4 Hz, ArH), 7.06 (2H, d, J = 8.4 Hz, ArH), 2.63 (2H, t, J = 7.8 Hz, CH2), 2.36 (2H, t, J = 7.2 Hz, CH2), 1 .97, (2H, dt, J = 7.2,7.8 Hz, CH2), OH not observed.
References:
WO2016/119017,2016,A1 Location in patent:Page/Page column 22; 31
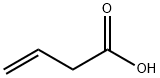
625-38-7
174 suppliers
$25.00/5g
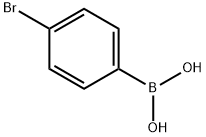
5467-74-3
470 suppliers
$8.00/1g

35656-89-4
128 suppliers
$25.00/1g
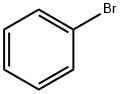
108-86-1
525 suppliers
$10.00/5g

35656-89-4
128 suppliers
$25.00/1g
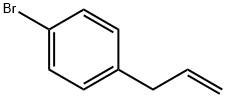
2294-43-1
41 suppliers
$256.00/1g

35656-89-4
128 suppliers
$25.00/1g

75906-36-4
43 suppliers
$110.00/0.5g

35656-89-4
128 suppliers
$25.00/1g