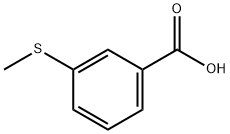
3-(Methylthio)benzoic acid synthesis
- Product Name:3-(Methylthio)benzoic acid
- CAS Number:825-99-0
- Molecular formula:C8H8O2S
- Molecular Weight:168.21
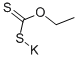
90721-40-7

825-99-0
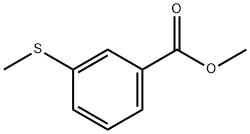
Methyl iodide (0.972 mL) was slowly added to a solution of DMF (8 mL) containing 3-mercaptobenzoic acid (601 mg, 3.9 mmol) and potassium carbonate (2.7 g, 19.5 mmol) under ice bath conditions. The reaction mixture was gradually warmed to room temperature and stirred continuously for 1 hour. After the reaction was completed, the reaction mixture was diluted with ethyl acetate, washed with water three times sequentially, and the organic phase was dried with anhydrous sodium sulfate, filtered and concentrated to give methyl 3-methylthiobenzoate (684 mg, 96%, yellow oil).1H NMR (CDCl3, δ ppm): 7.90 (s, 1H), 7.80 (d, 1H), 7.44 (d, 1H), 7.35 (t, 1H). 7.35 (t, 1H), 3.92 (s, 3H), 2.53 (s, 3H). Methyl 3-methylthiobenzoate (684 mg, 3.8 mmol) was dissolved in a mixed solvent of methanol (8 mL) and THF (8 mL), 1 N NaOH aqueous solution (5.6 mL, 5.6 mmol) was added, and the reaction was heated for 1 hr at 70 °C. After completion of the reaction, the reaction mixture was concentrated and the residue was diluted with water. After adjusting the pH to 2 with 1 N HCl, the aqueous phase was extracted with ethyl acetate, the organic phase was washed with water and saturated saline in turn, dried over anhydrous sodium sulfate, filtered and concentrated to give 3-methylthiobenzoic acid (616 mg, 97%, white solid).1H NMR (DMSO, δ ppm): 13.1 (bs, 1H), 7.76 (s, 1H), 7.70 (d, 1H ), 7.51 (d, 1H), 7.44 (t, 1H), 2.52 (s, 3H).

90721-40-7
9 suppliers
inquiry

825-99-0
107 suppliers
$17.00/1g
Yield:825-99-0 97%
Reaction Conditions:
Stage #1:3-methanesulfanyl benzoic acid methyl ester with sodium hydroxide;water in tetrahydrofuran;methanol at 70; for 1 h;
Stage #2: with hydrogenchloride in water; pH=2
Steps:
102 Example 102; 3-METHYLSULFANYL-BENZOIC acid
Methyl iodide (0.972 mL) was added to a mixture of 3-mercapto-benzoic acid (601 mg, 3.9 mmol) and potassium carbonate (2.7 g, 19.5 mmol) in DMF (8 mL) in an ice-bath. After the reaction was warmed to room temperature and stirred for 1 hour, the reaction mixture was diluted with ethyl acetate, washed with water (3X), dried over anhydrous sodium sulfate, filtered, and concentrated to afford 3-methylsulfanyl-benzoic acid methyl ester (684 mg, 96%, yellow [OIL). 1H] NMR [(CDC13),] [S] (ppm): 7.90 (s, 1H), 7.80 (d, [1H),] 7.44 (d, 1H), 7.35 (t, 1H), 3.92 (s, 3H), 2.53 (s, 3H). 3-Methylsulfanyl-benzoic acid methyl ester (684mg, 3.8 mmol) and 1N [NAOH] (5.6 mL, 5.6 mmol) in methanol (8 [ML)] and THF (8 mL) were heated at [70°C] for 1 hour. The reaction mixture was concentrated and then the residue was diluted with water. After acidification with 1N [HC1] to [PH-2,] the aqueous layer was extracted with ethyl acetate and then washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to afford 3-methylsulfanyl-benzoic acid (616 mg, 97%, white [SOLID). 1H] NMR (DMSO), [8] (ppm): 13.1 (bs, [1H),] 7.76 (s, 1H), 7.70 (d, 1H), 7.51 (d, 1H), 7.44 (t, 1H), 2.52 (s, 3H).
References:
ASTRA ZENECA AB;NPS PHARMACEUTICALS, INC. WO2004/14881, 2004, A2 Location in patent:Page 136
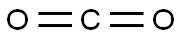
124-38-9
132 suppliers
$175.00/23402
33733-73-2
165 suppliers
$5.00/1g

825-99-0
107 suppliers
$17.00/1g

73771-35-4
46 suppliers
$65.00/50mg

825-99-0
107 suppliers
$17.00/1g