
3-Iodopyridin-2-amine synthesis
- Product Name:3-Iodopyridin-2-amine
- CAS Number:104830-06-0
- Molecular formula:C5H5IN2
- Molecular Weight:220.01
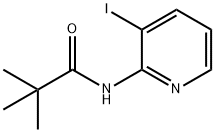
113975-31-8

104830-06-0
General procedure for the synthesis of 2-amino-3-iodopyridine from N-(3-iodopyridin-2-yl)palmitamide: N-(3-iodopyridin-2-yl)neopentanamide (13.80 g, 45.36 mmol) was dissolved in 24 wt% sulfuric acid solution (394 mL), and the reaction mixture was stirred for 60 min under reflux conditions. After completion of the reaction, it was cooled to room temperature and neutralized with 4N sodium hydroxide solution and solid sodium bicarbonate. The aqueous phase was extracted with dichloromethane (3 x 200 mL), the organic phases were combined and dried over anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporator to give 3-iodopyridin-2-amine (9.70 g, 97% yield) as a cream colored solid.
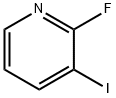
113975-22-7
311 suppliers
$7.00/1g

104830-06-0
235 suppliers
$7.00/250mg
Yield:104830-06-0 95%
Reaction Conditions:
with acetamidine hydrochloride;sodium hydroxide in water;dimethyl sulfoxide at 130; for 24 h;
Steps:
1 preparation of 3-iodo-2-aminopyridine
2-Fluoro-3-iodopyridine (1 mmol, 223.0 mg), acetamidine hydrochloride (1.2 mmol, 113.4 mg), NaOH (2.5 mmol, 100 mg), H2O (0.5 mL) and Dimethyl sulfoxide (2.5 mL). The reaction was carried out at 130 ° C for a reaction time of 24 hours. After the reaction was completed, it was cooled to room temperature.The reaction was quenched by adding 10 mL of ethyl acetate, and washed with 6 mL of saturated brine.The organic phase was separated, and the aqueous phase was extracted three times with ethyl acetate (6 mL of ethyl acetate each time). The organic phase was combined, dried over anhydrous sodium sulfate, and the solvent was removed by distillation under reduced pressure, including organic solvent and inorganic solvent. Organic solvent,The title product 3-iodopyridin-2-amine was isolated by column chromatography to give a yield of 95%.
References:
Wuyi University;Li Yibiao;Huang Guoling;Huang Shuo;Liao Chunshu;Zhong Zhengrong;Zhong Jingyi CN109232402, 2019, A Location in patent:Paragraph 0025; 0026

113975-31-8
81 suppliers
$24.00/1g

104830-06-0
235 suppliers
$7.00/250mg
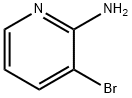
13534-99-1
365 suppliers
$4.00/1g

104830-06-0
235 suppliers
$7.00/250mg

78607-36-0
370 suppliers
$14.00/1g

104830-06-0
235 suppliers
$7.00/250mg
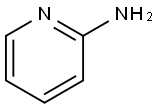
504-29-0
558 suppliers
$5.00/5 g

104830-06-0
235 suppliers
$7.00/250mg