
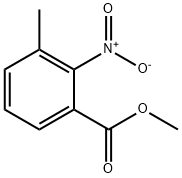
Methyl 3-formyl-2-nitrobenzoate synthesis
- Product Name:Methyl 3-formyl-2-nitrobenzoate
- CAS Number:138229-59-1
- Molecular formula:C9H7NO5
- Molecular Weight:209.16

132874-06-7

138229-59-1
Step C: Synthesis of methyl 3-formyl-2-nitrobenzoate; N-methylmorpholine oxide (NMO, 6.10 g, 52.07 mmol) was added to a solution of acetonitrile (150 mL) containing methyl 3-(bromomethyl)-2-nitrobenzoate (7.13 g, 26.023 mmol) and 4A molecular sieves (35.32 g) under nitrogen protection. The reaction mixture was stirred at room temperature for 1.5 hours. Subsequently, the reaction mixture was diluted with ethyl acetate (600 mL) and the insoluble material was removed by vacuum filtration. The filtrate was washed sequentially with water (100 mL), 1N HCl (100 mL) and brine (150 mL) and then dried with anhydrous sodium sulfate. The solvent was removed by concentration under reduced pressure and the residue was purified by fast column chromatography on silica gel, using ethyl acetate/hexane (1:3) as eluent to afford methyl 3-formyl-2-nitrobenzoate as an off-white solid (4.04 g, 74% yield). The structure of the product was confirmed by 1H NMR (CDCl3): δ 3.95 (s, 3H), 7.77 (t, 1H), 8.18 (dd, 1H), 8.28 (dd, 1H), 9.98 (s, 1H).

132874-06-7
31 suppliers
inquiry

138229-59-1
228 suppliers
$5.00/250mg
Yield:138229-59-1 85.2%
Reaction Conditions:
with 4-methyl-4-oxidomorpholin-4-ium in acetonitrile at 20; for 16 h;Molecular sieve;
Steps:
1 Compound 3:
Under the condition of 20, NMO (N-methylmorpholine-N-oxide) (85.5g, 730mmol, 77.0mL) was added in batches to the CH3CN (650mL) solution of compound 2 (100g, 365mmol, 1.00eq) , 2.00eq), the addition is completed within 30min, and then 4A molecular sieve (100g, 365mmol, 1.00eq) is added. The reaction mixture was stirred at 20°C for 16 h. TLC (petroleum ether: ethyl acetate=3:1, product: Rf=0.4) showed that the reaction was complete. The reaction solution was suction filtered, and the filter cake was washed three times with CH3CN (200 mL×3). The filtrate was added to water (1000 mL) and extracted. The aqueous phase was back-extracted twice with EtOAc (200 mL x 2), the organic phase was extracted with saturated brine, then the organic phase was dried over anhydrous Na2SO4, filtered with suction, and the filtrate was concentrated under vacuum. The residue was purified by a chromatographic column (SiO2, petroleum ether/ethyl acetate=100/10/1). Compound 3 (65.0 g, 311 mmol, yield 85.2%) was obtained as a pale white solid.
References:
CN112300074,2021,A Location in patent:Paragraph 0035; 0040-0041
5471-82-9
211 suppliers
$10.00/5g

138229-59-1
228 suppliers
$5.00/250mg
![3-[(E)-2-(Dimethylamino)ethenyl]-2-nitrobenzoic acid methyl ester](/CAS/GIF/68109-89-7.gif)
68109-89-7
14 suppliers
inquiry

138229-59-1
228 suppliers
$5.00/250mg

5437-38-7
481 suppliers
$5.00/10g

138229-59-1
228 suppliers
$5.00/250mg