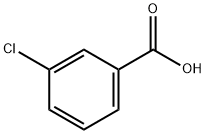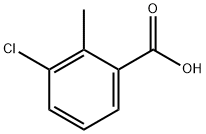
3-Chloro-2-methylbenzoic acid synthesis
- Product Name:3-Chloro-2-methylbenzoic acid
- CAS Number:7499-08-3
- Molecular formula:C8H7ClO2
- Molecular Weight:170.59

118-90-1

7499-08-3
7499-06-1

133232-56-1

54811-38-0
In a 100 mL three-necked flask equipped with a reflux condenser, 1.36 g (10 mmol) of 2-methylbenzoic acid was suspended in 25 mL of 30% (w/w) sulfuric acid. Subsequently, 2.4 g (15 mmol) of iodine monochloride was dissolved in 5 g of acetic acid and added slowly and dropwise to the reaction mixture over 40 min. The reaction was continued at 90°C for 5 hours. Upon completion of the reaction, the mixture was poured into 90 mL of water, the precipitate was collected by filtration and washed with aqueous sodium sulfite to give 1.6 g of crystalline solid product. Analysis of the product showed the following compositional distribution: 33% 2-methylbenzoic acid, 13% 5-chloro-2-methylbenzoic acid, 9% 3-chloro-2-methylbenzoic acid, 38% 5-iodo-2-methylbenzoic acid, 5% 3-iodo-2-methylbenzoic acid, and 2% other impurities. Attempts were made to purify the above mixture by recrystallization from acetic acid or isopropanol to isolate 5-iodo-2-methylbenzoic acid, but the purity of the mixture was not significantly improved, making it difficult to obtain pure 5-iodo-2-methylbenzoic acid.
608-23-1
119 suppliers
$25.00/5g
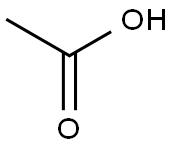
64-19-7
1602 suppliers
$10.00/25ML

7499-08-3
190 suppliers
$8.00/10g
Yield:7499-08-3 60%
Reaction Conditions:
with oxygen at 100 - 105; under 750.075 Torr; for 1 h;Autoclave;Temperature;Pressure;Reagent/catalyst;
Steps:
1
280 g of 3-chloro-o-xylene (2 mol), 28 g of acetic acid and 0.28 g ofcobalt-manganese bromide composite catalystwere added to a 500 ml autoclave(the catalyst included 0.136 g of cobalt acetate tetrahydrate, 0.105 g of manganese acetate tetrahydrate and0.039g ofpotassium bromide) , Stirring, heating to 100 ~ 105 .Slowly through the oxygen so that the pressure inside the kettle to0.1Mpa, oxygen into the way intermittent oxygen, the control of the internal temperature of 100 ~ 105 , about 1h pass.The concentrate wascooledto -0.095Mpa and the solvent was distilled off (the distillate was mainly acetic acid and can be recovered). The concentrate wascooled, filtered and dried to obtain 203.8 g of 2-methyl-3-chlorobenzoic acid, 98.5% purity,60%.
References:
Jiangsu Lianhua Technology Co., Ltd.;Lianhua Science And Technology (Yancheng) Co., Ltd.;Lianhua Science And Technology (Taizhou) Co., Ltd.;Fan Xiaobin;Lin Xingjun;Yang Wei CN106946685, 2017, A Location in patent:Paragraph 0040-0041; 0044; 0047; 0050; 0052

118-90-1
529 suppliers
$13.00/1g

7499-08-3
190 suppliers
$8.00/10g

7499-06-1
178 suppliers
$5.00/1g

133232-56-1
140 suppliers
$12.00/1g

54811-38-0
403 suppliers
$5.00/5g

146516-69-0
1 suppliers
inquiry

7499-08-3
190 suppliers
$8.00/10g

205178-79-6
34 suppliers
$26.00/1g

7499-08-3
190 suppliers
$8.00/10g