
3-Chloro-2-fluorobenzoic acid synthesis
- Product Name:3-Chloro-2-fluorobenzoic acid
- CAS Number:161957-55-7
- Molecular formula:C7H4ClFO2
- Molecular Weight:174.56
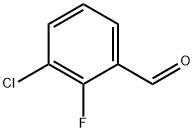
85070-48-0

161957-55-7
General procedure for the synthesis of 3-chloro-2-fluorobenzoic acid from 3-chloro-2-fluorobenzaldehyde: An acetone (75 mL) solution of 3-chloro-2-fluorobenzaldehyde (3 g, 18.92 mmol) was slowly added to an aqueous (30 mL) solution of sodium chlorite (6.81 g, 75.68 mmol) under cooling conditions in an ice bath. The reaction mixture was stirred at 0°C for 5 minutes. Subsequently, sulfamic acid (5.50 g, 56.76 mmol) was added all at once and stirring was continued for 30 min at the same temperature. The reaction process was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was diluted with water (5 mL) and filtered. The filtrate was extracted with ethyl acetate (3 x 50 mL), the organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give 3-chloro-2-fluorobenzoic acid as a white solid (3.2 g, 97% yield).LC-MS analysis: 172.9 (M-H).

85070-48-0
257 suppliers
$10.00/5g

161957-55-7
274 suppliers
$10.00/1g
Yield: 97%
Reaction Conditions:
Stage #1:2-fluoro-3-chlorobenzaldehyde with sodium chlorite in water;acetone for 0.0833333 h;Cooling with ice;
Stage #2: with aminosulfonic acid in water;acetone at 0; for 0.5 h;
Steps:
9H-fluoren-9-ylmethyl N-[[3-chloro-2-[(3-formyl-2-pyridyl)sulfanyl]phenyl]methyl]carbamate
To an ice-cooled solution of sodium chlorite (6.81 g, 75.68 mmol) in water (30 mL) was added a solution of 3-Chloro-2-fluoro-benzaldehyde (3 g, 18.92 mmol) in acetone (75 mL) and the reaction mixture was stirred for 5 mm. To the resulting reaction mixture was added sulphamic acid (5.50 g, 56.76 1 mmol) in one lot at 0 °C and stirring continued for 30 mm. Progress of the reaction was monitored by TLC. After completion, the reaction mixture wasdiluted with water (5 mL) and filtered. Filtrate was extracted with ethyl acetate (3 x 50 mL), combined organic layer was dried over anhydrous sodium sulphate, filtered and concentrated under reduced pressure to get 3-chloro-2-fluoro-benzoic acid (3.2 g, 97%) as white solid. LC-MS:172.9(M-H).
References:
F. HOFFMANN-LA ROCHE AG;HOFFMANN-LA ROCHE INC.;ALANINE, Alexander;BEIGNET, Julien;BLEICHER, Konrad;FASCHING, Bernhard;HILPERT, Hans;HU, Taishan;MACDONALD, Dwight;JACKSON, Stephen;KOLCZEWSKI, Sabine;KROLL, Carsten;SCHAEUBLIN, Adrian;SHEN, Hong;STOLL, Theodor;THOMAS, Helmut;WAHHAB, Amal;ZAMPALONI, Claudia WO2017/72062, 2017, A1 Location in patent:Page/Page column 166

94087-40-8
183 suppliers
$10.00/5g

161957-55-7
274 suppliers
$10.00/1g

348-51-6
221 suppliers
$21.00/5 g
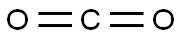
124-38-9
132 suppliers
$175.00/23402

161957-55-7
274 suppliers
$10.00/1g
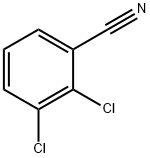
6574-97-6
220 suppliers
$6.00/1g

161957-55-7
274 suppliers
$10.00/1g