
3-CHLORO-1H-INDAZOL-5-AMINE synthesis
- Product Name:3-CHLORO-1H-INDAZOL-5-AMINE
- CAS Number:41330-49-8
- Molecular formula:C7H6ClN3
- Molecular Weight:167.6

4812-45-7

41330-49-8
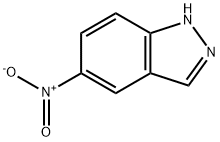
The general procedure for the synthesis of 3-chloro-1H-indole-5-aniline using 3-chloro-5-nitro-1H-indazole as starting material was as follows: 1.00 g (5.06 mmol) of 3-chloro-5-nitro-1H-indazole was suspended in 50 mL of ethanol, followed by the addition of 5.71 g (25.3 mmol) of tin chloride dihydrate. The reaction mixture was stirred under reflux conditions overnight. Upon completion of the reaction, saturated aqueous sodium bicarbonate was added to the mixture to neutralize the reaction system, followed by three extractions with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure to remove the solvent. The resulting crude product was ground with 10% butyl methyl ether and the solid product was collected by filtration. A final 544 mg of the target compound was obtained with a purity of 90% and a yield of 58% of the theoretical value.

4812-45-7
83 suppliers
$17.00/250mg

41330-49-8
60 suppliers
$65.00/100mg
Yield:41330-49-8 71%
Reaction Conditions:
with tin(II) chloride dihdyrate in ethanol; for 4 h;Reflux;
Steps:
To a solution of 3-chloro-5-nitro-lH-indazole (7) (310 mg, 1.57 mmol) in ethanol (150 mL) was added stannous chloride dihydrate (1.77 g, 7.85 mmol). The reaction mixture was refluxed for 4 h. After completion of reaction, the mixture was concentrated on rotary evaporator. The residue was diluted with dichloromethane and basified with sodium hydroxide. The mixture was transferred to separatory funnel and the aqueous layer was extracted with dichloromethane The combined organic layer was dried over anhydrous magnesium sulfate, fi tered, and concentrated in vacuo. The residue was purified by flash chromatography to provide 3-chloro-lH-indazol-5-amine (10) (186 mg, 71 yield), m/z 168 [M+H]+.
References:
WO2019/46467,2019,A1 Location in patent:Page/Page column 38
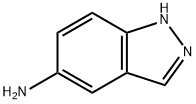
19335-11-6
313 suppliers
$10.00/1g

41330-49-8
60 suppliers
$65.00/100mg
5401-94-5
374 suppliers
$14.00/5g

41330-49-8
60 suppliers
$65.00/100mg

99-52-5
281 suppliers
$5.00/5G

41330-49-8
60 suppliers
$65.00/100mg