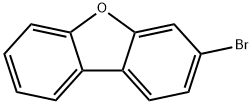
3-Bromodibenzo[b,d]furan synthesis
- Product Name:3-Bromodibenzo[b,d]furan
- CAS Number:26608-06-0
- Molecular formula:C12H7BrO
- Molecular Weight:247.09
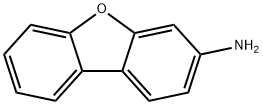
4106-66-5
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
The general procedure for the synthesis of 3-bromodibenzo[b,d]furan from 3-aminodibenzofuran was as follows: in a 500 mL four-necked round-bottomed flask equipped with a stirrer, a thermometer, and a 200 mL dropping funnel, 19.0 g (102.6 mmol) of 3-amino-dibenzofuran, 190 mL of water, and 57 mL of 48% hydrobromic acid were added, and the mixture was stirred overnight. Subsequently, the reaction mixture was cooled to -10°C in an ice-water bath and 8.1 g (117.4 mmol) of sodium nitrite solution dissolved in 150 mL of water was added slowly and dropwise while keeping the temperature from rising. The reaction solution was stirred below 5 °C for 1 h to prepare to obtain the diazonium salt solution. Next, in a 1 L four-necked round-bottomed flask equipped with a stirrer, condenser, thermometer, and 500 mL dropping funnel, 16.2 g (116.9 mmol) of cuprous bromide, 38 mL of 48% hydrobromic acid, and 90 mL of water were added, and the mixture was cooled to 0°C and stirred. The previously prepared diazonium salt solution was slowly added dropwise at no more than 5 °C and stirring was continued at the same temperature for 30 min. Subsequently, the reaction temperature was raised to 40°C and stirred at that temperature for 18 hours. Upon completion of the reaction, 200 mL of water was added to the reaction solution, cooled to room temperature and extracted twice with 200 mL of dichloromethane (DCM). The organic layers were combined, washed sequentially with 100 mL of 5% aqueous sodium sulfite and 100 mL of saturated brine, and dried with magnesium sulfate. After filtration to remove the desiccant, the solvent was removed by distillation under reduced pressure. Finally, the crude product was purified by silica gel column chromatography using n-heptane as the unfolding agent to afford 17.2 g (yield: 68.0%) of the target compound 3-bromodibenzo[b,d]furan.
![[1,1'-Biphenyl]-2-ol, 4'-bromo-2'-fluoro-](/CAS/20210305/GIF/1285719-49-4.gif)
1285719-49-4
1 suppliers
inquiry
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
193 suppliers
$8.00/250mg
Yield:26608-06-0 80%
Reaction Conditions:
with potassium carbonate in 1-methyl-pyrrolidin-2-one at 185; for 1.5 h;
Steps:
A-3 (A-3) Synthesis of Intermediate A-3
In argon atmosphere, a solution of Intermediate A-2 synthesized in the step (A-2) (561 g, 2.10 mol) and potassium carbonate (580 g, 4.20 mol) in N-methyl-2-pyrrolidone (NMP) (6 L) was stirred at 185° C. for 1.5 h. The reaction liquid was cooled and stirred after adding water. The generated solid matter was collected by filtration and washed with water. The obtained solid was dissolved into toluene and the insoluble was removed by filtration. The filtrate was concentrated. The crystal obtained by adding heptane was collected by filtration to obtain Intermediate A-3 (414 g). The yield was 80%.
References:
US2020/24263,2020,A1 Location in patent:Paragraph 0305; 0308

4106-66-5
133 suppliers
$10.00/250mg
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
193 suppliers
$8.00/250mg
5410-97-9
107 suppliers
$45.00/50mg
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
193 suppliers
$8.00/250mg

861605-94-9
1 suppliers
inquiry
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
193 suppliers
$8.00/250mg
![4'-Bromo-[1,1'-biphenyl]-2-ol](/CAS/20180713/GIF/21849-89-8.gif)
21849-89-8
29 suppliers
inquiry
![3-Bromodibenzo[b,d]furan](/CAS/GIF/26608-06-0.gif)
26608-06-0
193 suppliers
$8.00/250mg