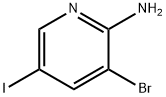
3-BROMO-5-IODOPYRIDIN-2-AMINE synthesis
- Product Name:3-BROMO-5-IODOPYRIDIN-2-AMINE
- CAS Number:697300-73-5
- Molecular formula:C5H4BrIN2
- Molecular Weight:298.91

20511-12-0

697300-73-5
Step 1: Synthesis of 3-bromo-5-iodopyridin-2-amine N-bromosuccinimide (NBS, 20.2 g, 113 mmol) was slowly added to a stirred solution of 5-iodopyridin-2-amine (25.0 g, 113 mmol) in acetonitrile (500 mL) at room temperature. After the addition was completed, the reaction mixture continued to be stirred at room temperature for 72 hours. Subsequently, the reaction solution was concentrated under reduced pressure at 40 °C to remove the solvent. The resulting residue was purified by column chromatography (silica gel, 200-300 mesh, eluent ethyl acetate/petroleum ether, 3:1, v/v) to afford the target product 3-bromo-5-iodopyridin-2-amine (15.9 g, 47% yield) as a yellow solid. The product was identified by 1H NMR (300 MHz, DMSO-d6): δ 8.07 (s, 1H), 7.98-7.97 (m, 1H), 6.43 (brs, 2H, NH2).The LC/MS analysis showed the molecular ion peak [M+H]+ as 298.9.
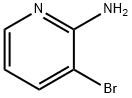
13534-99-1
366 suppliers
$4.00/1g

697300-73-5
135 suppliers
$33.00/250mg
Yield: 38%
Reaction Conditions:
with iodine in dimethyl sulfoxide at 20 - 100; for 16 h;
References:
González Cabrera, Diego;Douelle, Frederic;Younis, Yassir;Feng, Tzu-Shean;Le Manach, Claire;Nchinda, Aloysius T.;Street, Leslie J.;Scheurer, Christian;Kamber, Jolanda;White, Karen L.;Montagnat, Oliver D.;Ryan, Eileen;Katneni, Kasiram;Zabiulla, K. Mohammed;Joseph, Jayan T.;Bashyam, Sridevi;Waterson, David;Witty, Michael J.;Charman, Susan A.;Wittlin, Sergio;Chibale, Kelly [Journal of Medicinal Chemistry,2012,vol. 55,# 24,p. 11022 - 11030] Location in patent:supporting information

20511-12-0
467 suppliers
$6.00/1g

697300-73-5
135 suppliers
$33.00/250mg
