
3-BROMO-5-(2-PYRROLIDINYL)PYRIDINE synthesis
- Product Name:3-BROMO-5-(2-PYRROLIDINYL)PYRIDINE
- CAS Number:71719-06-7
- Molecular formula:C9H11BrN2
- Molecular Weight:227.1

64319-85-3

71719-06-7
General procedure for the synthesis of 3-bromo-5-(2-pyrrolidinyl)pyridine from 3-bromo-5-(3,4-dihydro-2H-pyrrol-5-yl)pyridine: 3-bromo-5-(3,4-dihydro-2H-pyrrol-5-yl)pyridine (2.0 g, 8.8 mmol) was dissolved in 80 mL of a mixed solvent of 80:20 (v/v) methanol/acetic acid, and the reaction was carried out using a dry ice-acetonitrile bath The reaction mixture was cooled to -40 °C. Sodium borohydride (747 mg, 19.75 mmol) was added to the reaction mixture in batches under vigorous stirring, and the addition process was controlled to be completed within 10 min. During the addition process, the reaction temperature was increased to -20 °C. After the reaction mixture warmed up to room temperature, most of the solvent was removed using a rotary evaporator. Water (200 mL) was added to the residue and the solution was adjusted to alkaline with sodium hydroxide. Subsequently, extraction was performed with dichloromethane (2 x 90 mL). The organic phases were combined, washed with saturated saline, dried over anhydrous potassium carbonate, filtered and concentrated. The crude product was purified by silica gel column chromatography (10×4 cm) with eluent of 1:1 (v/v) ethyl acetate-methanol to afford the target compound 3-bromo-5-(2-pyrrolidinyl)pyridine as a yellow oil in 1.8 g (90% yield). The product was structurally confirmed by NMR hydrogen (400 MHz, CDCl3) and carbon (100 MHz, CDCl3) spectra.

64319-85-3
13 suppliers
inquiry

71719-06-7
55 suppliers
$125.00/50mg
Yield:71719-06-7 90%
Reaction Conditions:
with sodium tetrahydroborate;acetic acid in methanol;acetonitrile at -40 - 20; for 0.166667 h;
Steps:
3-l3romo-5-(2-pyrrolidinyl)-pyridine (3):
A solution of compound 2 (2.0 g, 8.8 mmol) in 80 mE of 80:20 methanol/acetic acid was cooled at -40° C. with dry iceacetonitrile bath. To this reaction mixture sodium borohydride (747 mg, 19.75 mmol) was added portion wise over 10 mm with vigorous stirring. During the course of the addition, the temperature rose to -20° C. After warming to room temperature, most of the solvent was removed with a rotary evaporatot Water (200 mE) was added and the solution was made basic with NaOH and extracted with DCM (2x90 mE). The combined extracts were washed with brine, dried over K2C03, and evaporated. The residue was purified by flash chromatography on a silica gel column (10x4 cm) eluting with 1: 1 ethyl acetate-methanol afforded racemic 3 as a yellow oil: yield 1.8 g (90%); ‘H NMR (400 MHz, CDC13) ? 1.56-1.66 (m, 1H), 1.81-1.95 (m, 3H), 2.16-2.25 (m, 1H), 3.00-3.06 (m, 1H), 3.11-3.17 (m, 1H), 4.15 (t, 1H, J=7.6 Hz),7.88 (t, 1H, J=1.6 Hz), 8.46 (d, 1H, J=2.0 Hz) and 8.50 (d,1H, J=2.4 Hz); ‘3C NMR (100 MHz, CDC13) ? 25.6, 34.7,47.1, 59.3, 120.9, 136.8, 142.9, 146.8 and 149.2.
References:
US2016/375131,2016,A1 Location in patent:Paragraph 0082

201610-91-5
0 suppliers
inquiry

71719-06-7
55 suppliers
$125.00/50mg

20986-40-7
272 suppliers
$6.00/10g

71719-06-7
55 suppliers
$125.00/50mg
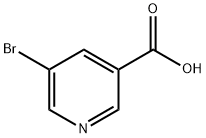
20826-04-4
554 suppliers
$5.00/1g

71719-06-7
55 suppliers
$125.00/50mg
![2-Pyrrolidinone, 3-[(5-bromo-3-pyridinyl)carbonyl]-1-ethenyl-](/CAS/20210305/GIF/201292-62-8.gif)
201292-62-8
0 suppliers
inquiry

71719-06-7
55 suppliers
$125.00/50mg