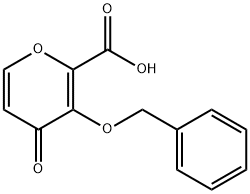
3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid synthesis
- Product Name:3-(Benzyloxy)-4-oxo-4h-pyran-2-carboxylic acid
- CAS Number:119736-16-2
- Molecular formula:C13H10O5
- Molecular Weight:246.22

Add 2-(hydroxymethyl)-3-(benzyloxy)-4H-pyran-4-one (Compound IV) 39.8Kg, dichloromethane (340L) and water (170L) to the reaction kettle to start stirring. Then add sodium bicarbonate (77Kg), sodium bromide (1.6Kg) and 2,2,6,6-tetramethylpiperidine-nitrogen-oxide (TEMPO) (2.3Kg), cool to 5℃, drop Add 10% sodium hypochlorite (340Kg). After the dropwise addition, stir at 5°C for 1 hour. The reaction is completed. Add 30% aqueous sodium thiosulfate solution (242L) and stir for 1 h. Add concentrated hydrochloric acid to adjust pH=4, separate the organic phase, extract the aqueous phase twice with dichloromethane (120L), combine the organic phases, and concentrate under reduced pressure to obtain the crude product. The crude product was recrystallized from acetonitrile to obtain 3-(benzyloxy)-4-oxo-4H-pyran-2-carboxylic acid (Compound VI) 32.4Kg, purity 99%, yield 77%. LC-MS: [M+H] + = 247.06, [M] + = 245.05; 1 H NMR (DMSO-d 6 ) δ8.00 (d, J = 5.6 Hz, 1H), 7.17 (s, 5H), 6.34 (d, J = 5.7 Hz, 1H), 4.91 (s, 2H), 2.30 (s, 1H).
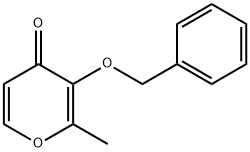
61049-69-2
88 suppliers
$68.00/25 mg

119736-16-2
262 suppliers
$68.00/5g
Yield:119736-16-2 71%
Reaction Conditions:
with chromium(VI) oxide;periodic acid in acetonitrile at 20; for 1.5 h;
Steps:
1.2
(2) adding 50 g of periodic acid to 350 mL of acetonitrile, stirring and dissolving,0.2 g of chromium trioxide and 20 g of intermediate 2 were sequentially added to the system.Stir at room temperature for 1.5 hours.The reaction is over.The solvent was evaporated to dryness, and 100 mL of dichloromethane and 200 mL of water were added to the residue.After the residue is dissolved, the organic phase is separated.The aqueous phase was extracted with dichloromethane (50 mL×2).Combine the organic phase,The organic phase was washed sequentially with saturated sodium bisulfite solution (50 mL) and saturated sodium chloride solution (50 mL).The organic phase is dry,Concentration gave 16 g of a white solid, Intermediate 3. Yield: 71%.
References:
FUREN PHARMACEUTICAL GROUP XIDELONG CANCER DRUG CO LTD;Furen Pharmaceutical Group Xidelong Tumor Medicine Co., Ltd.;KAIFENG PHARMACEUTICAL GROUP CO LTD;Kaifeng Pharmaceutical (Group) Co., Ltd.;HENAN FUREN MEDICAL TECH DEVELOPMENT CO LTD;Henan Furen Pharmaceutical Development Co., Ltd.;LI XUGUANG;Li Xuguang;CHEN SHUIKU;Chen Shuiku;ZHU SONGLIN;Zhu Songlin;ZHANG FANGJIE;Zhang Fangjie;FANG SHUIXIA;Fang Shuixia;LIU CONGJUN;Liu Congjun;ZHU HUIFENG;Zhu Huifeng;LUO QI;Luo Qi;MENG QINGLE;Meng Qingle;CUI HAO;Cui Hao CN108299466, 2018, A Location in patent:Paragraph 0032; 0034
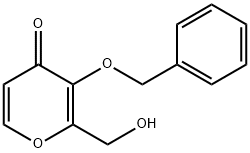
119736-15-1
0 suppliers
inquiry

119736-16-2
262 suppliers
$68.00/5g
![2-[(E)-2-phenylethenyl]-3-[(phenylmethyl)oxy]-4H-pyran-4-one](/CAS/20180703/GIF/1206102-05-7.gif)
1206102-05-7
17 suppliers
$140.00/25mg

119736-16-2
262 suppliers
$68.00/5g

500371-01-7
52 suppliers
inquiry

119736-16-2
262 suppliers
$68.00/5g
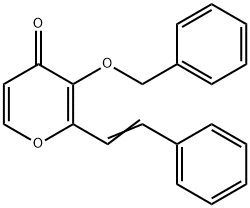
1229006-11-4
1 suppliers
inquiry

119736-16-2
262 suppliers
$68.00/5g