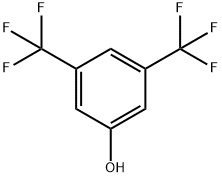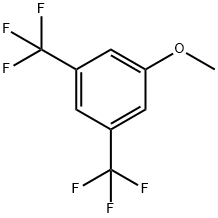
3,5-BIS(TRIFLUOROMETHYL)ANISOLE synthesis
- Product Name:3,5-BIS(TRIFLUOROMETHYL)ANISOLE
- CAS Number:349-60-0
- Molecular formula:C9H6F6O
- Molecular Weight:244.13

328-70-1
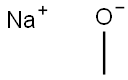
124-41-4

349-60-0
The general procedure for the synthesis of 3,5-bis(trifluoromethyl)anisole from 3,5-bis(trifluoromethyl)bromobenzene and sodium methanol was as follows: 25 g of 1-bromo-3,5-bis(trifluoromethyl)benzene (85 mmol) was mixed with 366 mg of copper(I) bromide (CuBr, 3 mol%), 1.57 g of 18-crown-6 (7 mol%), and 92.3 g of sodium methanol in methanol ( 30% concentration, 21% precursor concentration) were mixed and heated to 105 °C. The reaction conversion was >97% as determined by GC (total reaction time 22 h). Subsequently, the reaction mixture was poured into 175 g of water and the pH was adjusted to 5-6 by dropwise addition of hydrochloric acid and then filtered through diatomaceous earth. The aqueous phase was extracted twice with 150 g of dichloromethane. The organic phases were combined and purified by vacuum fractional distillation to afford 16.5 g of 3,5-bis(trifluoromethyl)anisole (68 mmol, 79.4% yield) with a GC purity of >98.5%.

328-70-1
441 suppliers
$10.00/25 g
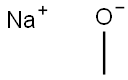
124-41-4
724 suppliers
$12.00/25g

349-60-0
112 suppliers
$9.00/1g
Yield: 79.4%
Reaction Conditions:
18-crown-6 ether;copper(I) bromide in methanol at 105; for 22 h;
Steps:
3
EXAMPLE 3 Preparation of 3,5-bis(trifluoromethyl)anisole From 1-bromo-3,5-bis(trifluoromethyl)benzene. 25 g of 1-bromo-3,5-bis(trifluoromethyl)benzene (85 mmol) are introduced into a mixture of 366 mg of copper(I) bromide (CuBr, 3 mol %), 1.57 g of 18-crown-6 (7 mol %) and 92.3 g of sodium methanolate solution in methanol (30% strength) (precursor concentration 21%) and heated to 105° C. After the conversion, determined by GC, it is >97% (total of 22 h), the reaction mixture is added to 175 g of water. The mixture is brought to pH 5-6 by metering in hydrochloric acid and is then filtered through decalite. The mixture if extracted twice with 150 g of dichloromethane each time. Vacuum fractionation of the combined organic phases results in 16.5 g of 3,5-bis(trifluoromethyl)anisole (68 mmol, 79.4%), GC purity >98.5% a/a.
References:
MEUDT, Andreas;Rausch, Bernhard J. US2008/71084, 2008, A1 Location in patent:Page/Page column 4

46331-50-4
126 suppliers
$11.00/250mg

349-60-0
112 suppliers
$9.00/1g
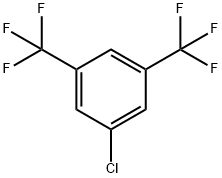
328-72-3
83 suppliers
$11.00/100mg
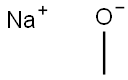
124-41-4
724 suppliers
$12.00/25g

349-60-0
112 suppliers
$9.00/1g
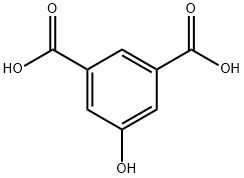
618-83-7
312 suppliers
$9.00/10g

349-60-0
112 suppliers
$9.00/1g