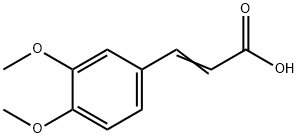
3,4-Dimethoxycinnamic acid synthesis
- Product Name:3,4-Dimethoxycinnamic acid
- CAS Number:2316-26-9
- Molecular formula:C11H12O4
- Molecular Weight:208.21

141-82-2

120-14-9

2316-26-9
GENERAL METHOD: In a dry reaction vessel, veratraldehyde (500 mg, 1.0 eq.) and malonic acid (981 mg, 2.0 eq.) were dissolved in dimethylformamide (5 mL), followed by the addition of DABCO (105 mg, 0.2 eq.). The reaction mixture was stirred at 100-110°C for 60-90 min. The reaction progress was monitored by thin layer chromatography (TLC). After completion of the reaction, the mixture was slowly poured into ice water and extracted with ethyl acetate (3 x 10 mL). The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by recrystallization from a chloroform/hexane solvent mixture to afford 3,4-dimethoxycinnamic acid in good yield.
Yield:2316-26-9 96%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane in N,N-dimethyl-formamide at 100 - 110; for 1.25 h;Knoevenagel-Doebner-Stobbe Reaction;
Steps:
Preparation of cinnamic acids:
General procedure: To aromatic aldehyde (500 mg, 1.0 equiv) and malonic acid (981 mg, 2.0 equiv) was added DABCO (105 mg, 2 equiv) in dimethyl formamide (5 mL) and the reaction mixture was stirred at 100-110 °C for 60-90 min. After completion of the reaction (TLC monitoring), reaction mixture was poured in water and then extracted with ethyl acetate. Crude product was recrystallized from chloroform/hexane system to provide cinnamic acids in good yield.
References:
Nagalakshmi;Diwakar;Govindh;Gopal Reddy;Venu;Bhargavi;Prasanna Devi;Murthy;Siddaiah [Asian Journal of Chemistry,2017,vol. 29,# 7,p. 1561 - 1564]

120-14-9
793 suppliers
$5.00/25g

2316-26-9
401 suppliers
$10.00/5g

20583-78-2
20 suppliers
inquiry

2316-26-9
401 suppliers
$10.00/5g

331-39-5
726 suppliers
$5.00/100mg

2316-26-9
401 suppliers
$10.00/5g
