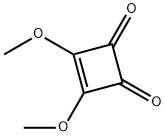
3,4-Dimethoxy-3-cyclobutene-1,2-dione synthesis
- Product Name:3,4-Dimethoxy-3-cyclobutene-1,2-dione
- CAS Number:5222-73-1
- Molecular formula:C6H6O4
- Molecular Weight:142.11
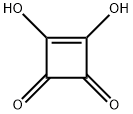
2892-51-5

149-73-5

5222-73-1
3,4-Dimethoxy-3-cyclobutene-1,2-dione (compound 30) was synthesized using a modified Liu et al. method [7]. The procedure was as follows: 3,4-dihydroxy-3-cyclobutene-1,2-dione (squaric acid, 2.053 g, 18 mmol) was dissolved in anhydrous methanol (18 mL), followed by addition of trimethyl orthoformate (4 mL, 36.5 mmol) to the solution. The reaction mixture was refluxed at 56 °C for 24 hours. Upon completion of the reaction, the crude product was concentrated under reduced pressure. The resulting light yellow solid was dissolved in dichloromethane and purified by silica gel column chromatography (eluent ratio of ethyl acetate: hexane = 1:2) to afford 3,4-dimethoxycyclobut-3-ene-1,2-dione (2.29 g, 89.4% yield) as a final white solid. The melting point of the compound is 55-57 °C (literature value 56-58 °C) [7,8]. NMR hydrogen spectrum (300 MHz, CD2Cl2) δ 4.34 (6H, s, OCH3); NMR carbon spectrum (75 MHz, CD3OD) δ 189.35 (C2, C=O), 189.35 (C1, C=O), 184.35 (C3), 184.35 (C4), 60.29 (OCH3), 60.29 ( OCH3). Low resolution mass spectra (EI): m/z 142.03 (100%), 114.03 (18%), 99.01 (16%), 86.01 (54%), 67.99 (8%), 56.25 (7%).
Yield:5222-73-1 87%
Reaction Conditions:
with sulfuric acid for 24 h;Reflux;
Steps:
2.2.1. Obtaining of 3,4-dimethoxy-3-cyclobutene-1,2-dione (4)
A solution of 0.30 g (2.6 mmol) of squaric acid (1) and 0.80 g(4.1 mmol) of concentrated sulfuric acid in 25 mL of anhydrous methanolin a round-bottomed flask equipped with a reflux condenser washeated until reflux for 24 h. After the reaction was complete, the solutionwas cooled, neutralized with saturated sodium bicarbonate solutionand extracted with 3 × 50 mL of dichloromethane. The organicphase was then dried with anhydrous magnesium sulfate, filtered andevaporated to afford 0.32 g (87%) of a yellow oil. IR (ATR, cm-1): 2996(νc-H), 1809 (νC=O), 1638 (νC=C), 1266 (νC-O), 735 (δC-H)·1H NMR(400 MHz, CDCl3-d1) δ 4.37. 13C NMR (400 MHz, CDCl3-d1) δ 61.22,184.67, 189.39.
References:
ávila-Costa, Marina;Donnici, Claudio L.;dos Santos, Jordana Dias;Diniz, Renata;Barros-Barbosa, Alexandre;Cuin, Alexandre;de Oliveira, Luiz Fernando Cappa [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2019,vol. 223,art. no. 117354]

67-56-1
782 suppliers
$7.29/5ml-f
61699-62-5
129 suppliers
$5.00/1g

5222-73-1
203 suppliers
$8.00/1g

