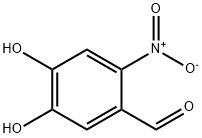
3,4-DIHYDROXY-6-NITROBENZALDEHYDE synthesis
- Product Name:3,4-DIHYDROXY-6-NITROBENZALDEHYDE
- CAS Number:73635-75-3
- Molecular formula:C7H5NO5
- Molecular Weight:183.12

73635-74-2

73635-75-3
An aluminum chloride solution was prepared by dissolving pure aluminum chloride (11 g, 82.4 mmol) in anhydrous 1,2-dichloroethane (25 mL) under argon protection and cooling to -5°C. Subsequently, Compound 1 (5 g, 25.6 mmol) was dissolved in anhydrous 1,2-dichloroethane (20 mL) and slowly added dropwise to the above aluminum chloride solution, keeping the temperature between -5°C and 5°C. The reaction was stirred continuously for 2 hours until the complete disappearance of compound 1 was confirmed by TLC detection. Upon completion of the reaction, the mixture was poured into 48% hydrobromic acid (60 mL) and stirred for 48 hours at room temperature (~20°C) until the intermediate chloromethyl ether disappeared (TLC monitoring). The reaction mixture was diluted with water (60 mL) and extracted with ethyl acetate (3 x 50 mL), the organic phases were combined and dried with magnesium sulfate. After evaporation of the solvent, the residue was treated with hot hexane. The crude product was further treated with hot dichloromethane (50 mL) to afford 4,5-dihydroxy-2-nitrobenzaldehyde (4.3 g, 90% yield). The product was recrystallized by water (100 mL) to afford chromatographically pure yellow crystals (1.79 g) with a melting point of 202-203 °C and an Rf value of 0.25 (acetone-hexane, 1:2).1H NMR (CDCl3) data: δ 7.21 (s, 1H, C6-H), 7.50 (s, 1H, C3-H), 10.14 (s, 1H, CHO) , 10.85 (s, 2H, 2×OH). 4,5-Dihydroxy-2-nitrobenzaldehyde (0.5 g, 2.7 mmol) was dissolved in DMF and anhydrous finely powdered potassium carbonate (0.94 g, 6.8 mmol) and n-bromobutane (1.1 g, 8.1 mmol) were added. The mixture was stirred under argon protection and protected from light, and the mixture was heated at 60 °C for 15 hours. After completion of the reaction, it was diluted with water and extracted with ether (3 x 50 mL). The organic phases were combined, dried with magnesium sulfate and the solvent was evaporated. The residue was recrystallized by ether to give chromatographically pure compound 5 (0.66 g, 83% yield) as a crystalline powder with a melting point of 80-85 °C and an Rf value of 0.62 (acetone-hexane, 1:2). Elemental analysis measured values (%): C, 61.04; H, 7.20; N, 5.71. molecular formula C15H21NO5 calculated values (%): C, 61.00; H, 7.17; N, 4.74. 1H NMR (CDCl3) data: δ 0.98-1.03 (m, 6H, 2 × CH3), 1.47-1.58 (m, 4H, 2 × CH2), 1.82-1.91 (m, 4H, 2 × CH2), 4.13-4.16 (m, 4H, 2 × CH2), 7.38 (s, 1H, C6-H), 7.58 (s, 1H, C3-H), 10.43 (s, 1H, CHO).
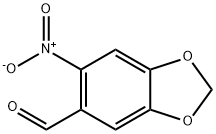
712-97-0
119 suppliers
$16.00/1 g

73635-75-3
47 suppliers
inquiry
Yield:73635-75-3 77.4%
Reaction Conditions:
Stage #1:6-nitro-1,3-benzodioxole-5-carbaldehyde with aluminum (III) chloride for 2 h;Cooling with ice;
Stage #2: with hydrogen bromide at 20; for 48 h;
Steps:
1.2 Demethylene reaction
Dissolve 1 g of 6-nitropiperonal in 10 mL of organic solvent.It was added dropwise to 2.5 g of an aluminum chloride organic solvent, and reacted for 2 hours under ice bath.After completion of the reaction, the reaction solution was poured into 25 mL of hydrobromic acid, and stirred at room temperature for 48 h.The reaction mixture was diluted with a large amount of water and extracted with ethyl acetate three times.Drying over anhydrous magnesium sulfate, the filtrate obtained by suction filtration was dried.Recrystallization from solvent and suction filtration to obtain yellow pure 4,5-dihydroxy-2-nitrobenzaldehyde0.72g,The yield was 77.4%.
References:
Dalian University of Technology;Qiao Weihong;Yao Weihe;Wang Ning CN110003031, 2019, A Location in patent:Paragraph 0038; 0041; 0042
2426-87-1
250 suppliers
$8.00/5g

73635-75-3
47 suppliers
inquiry
121-33-5
1167 suppliers
$9.00/1g

73635-75-3
47 suppliers
inquiry

2454-72-0
57 suppliers
inquiry

73635-75-3
47 suppliers
inquiry