
2-(TRIBUTYLSTANNYL)THIOPHENE synthesis
- Product Name:2-(TRIBUTYLSTANNYL)THIOPHENE
- CAS Number:54663-78-4
- Molecular formula:C16H30SSn
- Molecular Weight:373.18
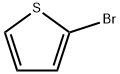
1003-09-4
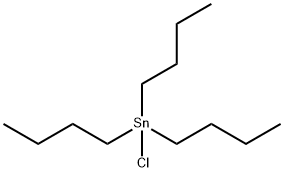
1461-22-9

54663-78-4
2-Bromothiophene (4.89 g, 30 mmol) and 60 mL of anhydrous tetrahydrofuran were added to a 250 mL three-necked flask with a device constant pressure dropping funnel. The system was evacuated and then displaced three times with argon to remove air. The reaction flask was placed in a cryostat reaction bath and cooled to -20 °C. Subsequently, a n-hexane solution of n-butyllithium (2.2 mol/L, 15 mL, 33 mmol) was slowly added dropwise under argon protection and the temperature was maintained at -20 °C for 1 h of reaction. Next, tributyltin chloride (8.95 mL, 33 mmol) was slowly added dropwise and the reaction was continued at -20 °C for 1 hour. Upon completion of the reaction, the reaction system was slowly brought to room temperature and stirred overnight. After the reaction was terminated, 150 mL of deionized water was added to the reaction flask and extracted three times with dichloromethane (80 mL x 3). The organic layers were combined and washed three times with saturated saline (100 mL x 3). The organic phase was dried overnight over anhydrous magnesium sulfate and filtered to remove the desiccant. The filtrate was concentrated by rotary evaporation and the residue was purified by silica gel column chromatography using a petroleum ether solution containing a small amount of triethylamine as eluent to give 11.08 g of the colorless liquid product 2-tributylmethylstannylthiophene in 90% yield.

1003-09-4
488 suppliers
$10.00/25g

1461-22-9
218 suppliers
$10.00/10g

54663-78-4
143 suppliers
$25.00/5g
Yield:54663-78-4 90%
Reaction Conditions:
Stage #1:2-bromothiophene with n-butyllithium in tetrahydrofuran;hexane at -20; for 1 h;Inert atmosphere;
Stage #2:tributyltin chloride in tetrahydrofuran;hexane at -20 - 20;Inert atmosphere;
Steps:
1.1 (1) Synthesis of Intermediate 1
In three-necked 250mL flask were added 2-bromothiophene (4.89g, 30mmol) and 60mL of anhydrous tetrahydrofuran, and put on a constant pressure funnel, evacuated, purged with argon, the reaction flask was placed in cryostat reactor cooled to -20 deg. C, then added dropwise n-butyllithium (15mL, 33mmol) in n-hexane (2.2mol / L), the reaction was continued at -20 deg. C for 1h, then slowly added dropwise (8.95mL, 33mmol) tributyltin chloride, after the reaction 1h, the reaction flask was moved at room temperature overnight. The reaction was stopped, the reaction flask was added 150mL of deionized water, and extracted three times with dichloromethane (80mL × 3), then the organic layer was washed three times with saturated brine (100mL × 3), combined organic phase was dried over anhydrous magnesium sulfate overnight . The filtrate was collected by filtration, solvent was removed by rotary evaporation, and the residue was mixed with a small amount of triethylamine in petroleum ether as eluent to silica gel column chromatography, to give a colorless liquid 11.08g, 90% yield.
References:
Dongguan University of Technology;Liao, Junxu;Zhao, Hongbin;Han, Lifen;Peng, Zaixi;Zhang, Wentao;Liu, Chuansheng;Peng, Fei;Zong, Qiao CN105732680, 2016, A Location in patent:Paragraph 0021

188290-36-0
1 suppliers
inquiry

1461-22-9
218 suppliers
$10.00/10g

54663-78-4
143 suppliers
$25.00/5g

1461-22-9
218 suppliers
$10.00/10g

54663-78-4
143 suppliers
$25.00/5g

1003-09-4
488 suppliers
$10.00/25g

813-19-4
174 suppliers
$14.00/1g

54663-78-4
143 suppliers
$25.00/5g

1003-09-4
488 suppliers
$10.00/25g

1067-52-3
94 suppliers
$49.20/25g

54663-78-4
143 suppliers
$25.00/5g