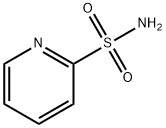
2-Pyridinesulfonamide(6CI,7CI,9CI) synthesis
- Product Name:2-Pyridinesulfonamide(6CI,7CI,9CI)
- CAS Number:63636-89-5
- Molecular formula:C5H6N2O2S
- Molecular Weight:158.18

2637-34-5

63636-89-5
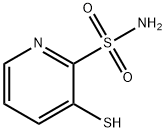
General procedure for the synthesis of pyridine-2-sulfonamide from 2-pyridinethione: To a 1000 mL round-bottomed flask was added dichloromethane (200 mL) and 1 M aqueous hydrochloric acid solution (200 mL, 200 mmol, 5.0 equiv.), and the suspension was cooled to -5 to -10 °C using an ice-salt bath. To the well-stirred suspension was added 2-mercaptopyridine (4.45 g, 40 mmol), and the resulting yellow mixture was stirred for 10 minutes, followed by the dropwise addition of 6% sodium hypochlorite solution (208 mL, 132 mmol, 3.3 eq.) over 5 minutes. After the dropwise addition, the mixture was continued to be stirred at the same temperature for 10 min and subsequently transferred to a separatory funnel for separation. The separated organic layer was immediately added dropwise to a pre-cooled and stirred mixture of saturated ammonia methanol solution (80 mL) and dichloromethane (80 mL) at 0 °C. The aqueous layer in the partition funnel was washed twice with dichloromethane, and the organic layer was combined and added dropwise to the ammonia solution. The resulting white suspension was slowly warmed to room temperature and stirred for 2 hours. Subsequently, the solvent was removed by evaporation to give a white solid. The solid was dissolved in hot ethyl acetate (250 mL x 2) and filtered through filter paper to remove ammonium chloride. The filtrate was evaporated to give the crude product, which was finally purified by recrystallization from ethyl acetate and hexane to give pure pyridine-2-sulfonamide (4.15 g, 70% yield). The product was characterized by 1H NMR (500 MHz, DMSO-d6) and 13C NMR (125 MHz, DMSO-d6) and the data were consistent with the expected structure.

16133-25-8
470 suppliers
$6.00/1g

63636-89-5
87 suppliers
$45.00/50mg
Yield:-
Steps:
Preparation of the starting compounds
Preparation of the starting compounds In analogy to Example 13, paragraph a), from 3-pyridylsulphonyl chloride (J Org. Chem., Vol. 54, 389) and ammonia there was obtained 2-pyridylsulphonamide as a white, crystalline solid, the potassium salt being obtained therefrom with potassium tert.-butylate in methanol.
References:
Hoffmann-La Roche Inc. US5837708, 1998, A

2637-34-5
322 suppliers
$6.00/5g

63636-89-5
87 suppliers
$45.00/50mg
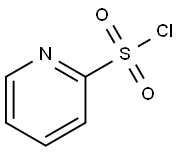
66715-65-9
140 suppliers
$117.00/50mg

63636-89-5
87 suppliers
$45.00/50mg
99780-72-0
3 suppliers
inquiry

352-91-0
124 suppliers
inquiry

63636-89-5
87 suppliers
$45.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
109-04-6
539 suppliers
$6.00/25g

63636-89-5
87 suppliers
$45.00/50mg