
2-Methyl-4-methoxybenzenamine synthesis
- Product Name:2-Methyl-4-methoxybenzenamine
- CAS Number:102-50-1
- Molecular formula:C8H11NO
- Molecular Weight:137.18
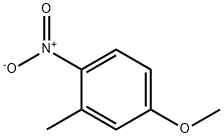
5367-32-8

102-50-1
GENERAL PROCEDURE: 3-methyl-4-nitroanisole (1.0 mmol), B2(OH)4 (5.0 eq., 5.0 mmol) and H2O (3.0 mL) were added to a 10 mL reaction tube. The reaction mixture was stirred at 80 °C for 8 hours. After completion of the reaction (monitored by TLC), the mixture was cooled to room temperature and extracted with ethyl acetate (3 x 20 mL). The organic phases were combined, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by silica gel column chromatography to give 2-methyl-4-methoxyaniline.

5367-32-8
186 suppliers
$10.00/5g

102-50-1
237 suppliers
$6.00/5g
Yield:102-50-1 80%
Reaction Conditions:
with tetrahydroxydiboron;water at 80; for 8 h;
Steps:
2. General procedure for the reduction of nitroarenes
General procedure: Nitro aromatic (1.0 mmol), B2(OH)4 (5.0 equiv, 5.0 mmol), and H2O (3.0 mL) were added in a10 mL tube. The reaction mixture was stirred at 80 °C for 8 h. When the reaction was completemonitored by TLC, the mixture was cooled to room temperature, extracted with ethyl acetate (3 ×20 mL). The combined organic phase was dried over anhydrous Na2SO4, filtered, andconcentrated under reduced pressure. The residue was purified by silica gel columnchromatography.
References:
Chen, Danyi;Zhou, Yanmei;Zhou, Haifeng;Liu, Sensheng;Liu, Qixing;Zhang, Kaili;Uozumi, Yasuhiro [Synlett,2018,vol. 29,# 13,p. 1765 - 1768] Location in patent:supporting information

27060-75-9
287 suppliers
$8.00/5g

102-50-1
237 suppliers
$6.00/5g
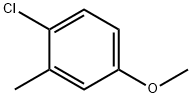
13334-71-9
97 suppliers
$25.00/5g

102-50-1
237 suppliers
$6.00/5g
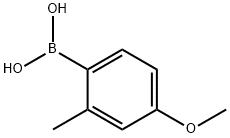
208399-66-0
190 suppliers
$8.00/1g

102-50-1
237 suppliers
$6.00/5g
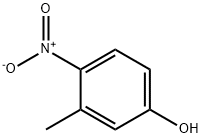
2581-34-2
262 suppliers
$10.00/5 g

102-50-1
237 suppliers
$6.00/5g