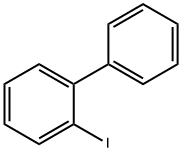
2-Iodobiphenyl synthesis
- Product Name:2-Iodobiphenyl
- CAS Number:2113-51-1
- Molecular formula:C12H9I
- Molecular Weight:280.1
Yield:2113-51-1 90%
Reaction Conditions:
Stage #1: 2-iodophenylamine;2-phenylanilinewith hydrogenchloride in tetrahydrofuran;water; for 0.333333 h;
Stage #2: with sodium nitrite in tetrahydrofuran;water; for 0.5 h;Cooling with ice;
Stage #3: with potassium iodide in tetrahydrofuran;water; for 1.5 h;Cooling with ice;
Steps:
1.1.2 2 - iodo - 1, 1' - biphenyl (1a - 2) preparation:
to the 1a - 1 of the tetrahydrofuran solution (280 ml) is added in the 4M hydrochloric acid aqueous solution (221.7 ml). Stirring 20 minutes, ice water bath under dropwise sodium nitrite (9.17g, 132 . 96mmol) aqueous solution (20 ml). Stirring 30 minutes later, in the ice water bath under dropping potassium iodide (36.79g, 221 . 16mmol) aqueous solution (20 ml). Stirring 30 minutes later, the solution temperature to room temperature slowly, stirring for one hour. Finally, dropping 1M of the sodium thiosulfate solution until the reaction solution unchanged color. Separating, the aqueous phase is extracted with ethyl acetate (100 ml × 3). The combined organic phase, it with water (50 ml × 2) and saturated salt water (50 ml × 1) washing, drying with anhydrous sodium sulfate, filtered, the solvent is removed under reduced pressure. Residue with silica gel column chromatography (eluant volume ratio, petroleum ether: ethyl acetate=20:1 - 10:1) purification, to obtain the colorless liquid 1a - 2 (22.34g, 90% yield).
References:
CN106905133,2017,A Location in patent:Paragraph 0060; 0061; 0063

90-41-5
356 suppliers
$16.00/5g

2113-51-1
265 suppliers
$6.00/1g
947-84-2
301 suppliers
$6.00/5g

2113-51-1
265 suppliers
$6.00/1g
615-42-9
289 suppliers
$6.00/1g
71-43-2
718 suppliers
$11.19/25ML
84-15-1
173 suppliers
$6.00/5g

2113-51-1
265 suppliers
$6.00/1g

