
2-Iodobenzyl bromide synthesis
- Product Name:2-Iodobenzyl bromide
- CAS Number:40400-13-3
- Molecular formula:C7H6BrI
- Molecular Weight:296.93
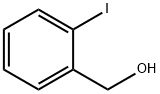
5159-41-1

40400-13-3
General procedure for the synthesis of 2-iodobenzyl bromide from 2-iodobenzyl alcohol: 48% hydrobromic acid (10 mL) and 98% sulfuric acid (2 mL) were added to 2-iodobenzyl alcohol (0.94 g, 4.0 mmol). The resulting suspension was placed in an oil bath and heated and stirred at 150 °C for 2 hours. After completion of the reaction, the mixture was cooled to room temperature and extracted with dichloromethane (2 x 10 mL). The organic layers were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure to give the crude product. The crude product was purified by fast column chromatography (silica gel, 0-20% dichloromethane/hexane gradient elution) to afford the target compound 2-iodobenzyl bromide (1.0 g, 84% yield).
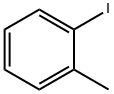
615-37-2
294 suppliers
$5.00/5g

40400-13-3
177 suppliers
$21.00/1G
Yield:40400-13-3 69%
Reaction Conditions:
with N-Bromosuccinimide;2,2'-azobis(isobutyronitrile) in tetrachloromethaneReflux;
Steps:
1-(bromomethyl)-2-iodobenzene
A mixture of l-iodo-2-methylbenzene (0.5 g, 2.3 mmol), N-bromosuccinimide (0.7 g, 3.7 mmol) and 2,2'-azobisisobutyronitrile (0.02 g, 0.1 mmol) in tetrachloromethane (10 mL) was heated at reflux until the reaction was completed (tic control). After cooled down to the ambient temperature the reaction mixture was quenched with water and water layer was extracted with dichloromethane. Combined organic phases were washed with sodium hydrocarbonate, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography (silica gel; hexane/ethyl acetate 9: 1) to give l-(bromomethyl)-2-iodobenzene (0.5 g) as yellowish oil; yield 69%.
References:
SELVITA S.A.;RZYMSKI, Tomasz;MILIK, Mariusz;BRZOZKA, Krzysztof;FABRITIUS, Charles-Henry;KUCWAJ-BRYSZ, Katarzyna;KULESZA, Urszula;WINCZA, Ewelina;DREAS, Agnieszka;GALEZOWSKI, Michal WO2017/68064, 2017, A1 Location in patent:Page/Page column 188; 189

5159-41-1
223 suppliers
$8.00/5g

40400-13-3
177 suppliers
$21.00/1G

18698-96-9
252 suppliers
$5.00/250mg

40400-13-3
177 suppliers
$21.00/1G
624-31-7
435 suppliers
$9.00/25g

40400-13-3
177 suppliers
$21.00/1G
