
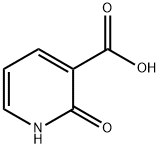
2-hydroxy-5-iodonicotinic acid synthesis
- Product Name:2-hydroxy-5-iodonicotinic acid
- CAS Number:390360-97-1
- Molecular formula:C6H4INO3
- Molecular Weight:265.01
609-71-2

390360-97-1
General procedure for the synthesis of 2-hydroxy-5-iodopyridine-3-carboxylic acid from 2-hydroxynicotinic acid: a mixture of 2-hydroxynicotinic acid (24 g, 173 mmol) and N-iodosuccinimide (50.5 g, 224 mmol) in N,N-dimethylformamide (DMF, 300 mL) was reacted for 1 h at room temperature, protected from light, and then stirred for 20 h at 50 °C. . Upon completion of the reaction, about 250 mL of DMF was removed by distillation under reduced pressure and water (400 mL) was added to the residue. The mixture was stirred at room temperature for 1 h, followed by continued stirring at 0 °C for 1 h. The precipitated solid was collected by filtration. The resulting solid was suspended in methanol (MeOH, 200 mL) and stirred at room temperature for 2 h. The mixture was filtered again, washed with methanol (40 mL), and finally dried under vacuum to afford the target compound 2-hydroxy-5-iodopyridine-3-carboxylic acid (19, light yellow solid, 38.4 g, 76% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3) δ= 8.41 (d, J = 2.4 Hz, 1H), 8.23 (d, J = 2.4 Hz, 1H) and mass spectra (ESI+) m/z 265.9 ([M + H]+).
78686-83-6
90 suppliers
$45.00/100mg

390360-97-1
43 suppliers
$72.00/500mg
Yield:390360-97-1 91 %
Reaction Conditions:
with lithium hydroxide monohydrate;water in tetrahydrofuran at 50;
Steps:
27.1 6-chloro-5-(5,7-difluoro-4-oxo-1,4-dihydroquinolin-2-yl)nicotinonitrile
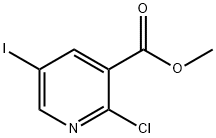
Step 1, 2-hydroxy-5-iodonicotinic acid: To a solution of methyl 2-chloro-5- iodonicotinate (3.0 g, 10.7 mmol, 1.0 equiv.) in THF (20 mL) and H2O (7 mL) was added LiOH·H2O (1.3 g, 32.2 mmol, 3.0 equiv.). The mixture was stirred at 50 °C for 16 hours. The mixture was concentrated under reduced pressure to remove THF. The pH of the mixture was adjusted to pH = 6 with aqueous 1 M HCl. During the pH adjustment a white solid precipitated and was collected by filtration. The filter cake was washed with H2O (10 mL) and concentrated in vacuum to give 2-hydroxy-5-iodonicotinic acid (2.5 g, 91% yield) as a white solid. LCMS [M+1] = 265.9.1H NMR (400 MHz, DMSO-d6) δ 8.28 (d, J = 2.4 Hz, 1H), 8.21 (d, J = 2.4 Hz, 1H).
References:
WO2023/14861,2023,A1 Location in patent:Paragraph 00115-00117

609-71-2
386 suppliers
$5.00/5g

390360-97-1
43 suppliers
$72.00/500mg