
2-Formylfuran-5-boronic acid synthesis
- Product Name:2-Formylfuran-5-boronic acid
- CAS Number:27329-70-0
- Molecular formula:C5H5BO4
- Molecular Weight:139.9
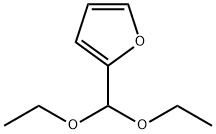
13529-27-6

27329-70-0
Under nitrogen protection, 20.16 g (0.118 mol) of 2-(diethoxymethyl)furan, 33.4 g (0.177 mol) of triisopropyl borate, and 40 mL of anhydrous tetrahydrofuran (THF) were added to a dry 500 mL three-necked flask equipped with a mechanical stirrer, an internal thermometer, and a dosing funnel. The water content of the reaction mixture was determined by Karl-Fischer titration to ensure that it was less than 800 μg/mL. the reaction mixture was cooled to an internal temperature of -10 °C. The reaction mixture was then purified to a temperature of -10 °C. The water content of the reaction mixture was determined by titration. With the temperature maintained at -10 °C to 0 °C, 84 mL (25 wt%, 1.84 M THF solution containing heptane and ethylbenzene from Chemmetal, content determined by titration, 1.3 equivalents) of LDA solution was slowly added through the addition funnel over a period of 1 hour. Upon completion of addition, the reaction mixture was transferred via cannula to a pre-cooled aqueous hydrochloric acid solution (prepared by mixing 33 mL of concentrated hydrochloric acid and 55 mL of water). The reaction temperature was controlled not to exceed 30 °C. The resulting tan slurry of 5-formylfuran-2-boronic acid was cooled to 0°C and filtered. The filter cake was washed twice with 20 mL of cold water to obtain 17.6 g of wet filter cake. The wet filter cake was dried in a vacuum oven at 40 °C to give 12.41 g of an off-white product, 5-formylfuran-2-boronic acid, in a total yield of 75%. The amount of unreacted furfural in the crude product was determined to be less than 0.1% by analysis.

13529-27-6
165 suppliers
$36.97/5G

27329-70-0
475 suppliers
$6.00/1g
Yield:27329-70-0 75%
Reaction Conditions:
Stage #1:2-(diethoxymethyl)furan with Triisopropyl borate;lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -10 - 0; for 1 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;n-heptane;ethylbenzene;water at 30;
Steps:
Example
A dried 500 mL 3-neck flask with mechanical stirrer, internal thermometer and addition funnel under nitrogen is charged with 20,16 g (0,118 mol) furfuraldiethylacetal, 33,4 g (0,177 mol) triisopropylborate and 40 mL of anhydrous THF. The water content of the reaction mixture was measured according to the Karl-Fischer-titration method and was determined to be less than 800 μg/mL of water. The solution is cooled to an internal temperature of -10°C. Keeping the temperature at -10°C to 0°C 84 mL (25 wt%, 1.84 M solution THF, heptane, ethylbenzene from Chemmetal, content determined by titration, 1.3 equivalents) LDA was added via addition funnel to the reaction mixture over a period of 1 hour. Using a canula the reaction mixture was subsequently transferred to precooled aqueous hydrochloric acid which was obtained by mixing 33 mL of concentrated hydrochloric acid and 55 mL water. The reaction temperature was maintained at a temperature of less than 30°C. The resulting tan slurry of 5-formyl-2-furylboronic acid was cooled to 0°C and filtered. The filter cake was washed twice with 20 mL of cold water to give 17.6 g of wet cake. Drying (40°C, vacuum oven) gave 12.41 g of an off-white product. The overall yield of 5-formyl-2-furylboronic acid was 75%. The content of unreacted furfural in the crude product was determined to be less than 0,1%.
References:
Degussa AG EP1403271, 2004, A1 Location in patent:Page 3, 4

13529-27-6
165 suppliers
$36.97/5G
121-43-7
385 suppliers
$13.00/25mL

27329-70-0
475 suppliers
$6.00/1g

1708-41-4
52 suppliers
$45.00/250mg

27329-70-0
475 suppliers
$6.00/1g
1899-24-7
270 suppliers
$13.43/500mgs:

5419-55-6
391 suppliers
$12.00/5g

27329-70-0
475 suppliers
$6.00/1g