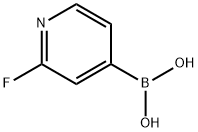
2-Fluoropyridine-4-boronic acid synthesis
- Product Name:2-Fluoropyridine-4-boronic acid
- CAS Number:401815-98-3
- Molecular formula:C5H5BFNO2
- Molecular Weight:140.91

5419-55-6

128071-98-7

401815-98-3
Step 1: 4-bromo-2-fluoropyridine (30.0 g, 0.17 mol) and triisopropyl borate (38.4 g, 0.20 mol) were dissolved in a solvent mixture of anhydrous toluene and tetrahydrofuran (THF) (4:1, 250 mL) under nitrogen protection. The reaction mixture was cooled to -78 °C, followed by slow dropwise addition of n-butyllithium (80 mL, 0.20 mol, 2.5 M hexane solution) over 30 min. After the dropwise addition was completed, stirring was continued at -78 °C for 30 min. The reaction mixture was then slowly warmed up to -20°C over 1 hr. The progress of the reaction was monitored by thin layer chromatography (TLC) (unfolding agent: petroleum ether (PE):ethyl acetate (EtOAc) = 1:1) to confirm that the raw materials were completely consumed. Upon completion of the reaction, the mixture was acidified to pH 2 with 3N HCl (50 mL) and stirred at room temperature for 15 minutes. The reaction mixture was transferred to a partition funnel and extracted with EtOAc (150 mL) and water (150 mL). The organic layer was separated, washed sequentially with water and brine, dried over anhydrous MgSO4, filtered and concentrated under reduced pressure to give (2-fluoropyridin-4-yl)boronic acid (22.0 g, 91% yield) as a white solid.

5419-55-6
391 suppliers
$12.00/5g

128071-98-7
293 suppliers
$7.00/1g

401815-98-3
251 suppliers
$10.00/250mg
Yield:401815-98-3 91%
Reaction Conditions:
with n-butyllithium in tetrahydrofuran;hexane;toluene at -78; for 1 h;Inert atmosphere;
Steps:
A.1
[00178] Step 1 : To a solution of anhydrous toluene and tetrahydrofuran ("THF") (4:1, 250 mL) were added 4-bromo-2-fluoropyridine 1 (30.0 g, 0.17 mol) and triisopropyl borate (38.4 g, 0.20 mol). The mixture was cooled to -78 °C under a nitrogen atmosphere. Then, n- butyllithium (80 mL, 0.20 mol) (2.5 M in hexanes) was added dropwise over 30 minutes, followed by stirring at the same temperature for an additional 30 minutes. The mixture was finally warmed up to -20 °C over 1 hour. Thin layer chromatography ("TLC") (petroleum ether ("PE"):ethyl acetate ("EtOAc") = 1 :1) indicated that the starting material was consumed. The reaction mixture was acidified to a pH of 2 with 3N HCl (50 mL) and then stirred at room temperature for 15 minutes. The mixture was partitioned between EtOAc (150 mL) and water (150 mL). The organic layer was isolated, washed with water, brine, dried over anhydrous MgS04, then filtered and evaporated to provide (2-fluoropyridin-4-yl)boronic acid (22.0 g, 91%) as a solid.
References:
ARRAY BIOPHARMA INC.;GENENTECH, INC.;BLAKE, James, F.;COOK, Adam;GAUDINO, John;GUNAWARDANA, Indrani, W.;HICKEN, Erik, James;HUNT, Kevin, W.;LYON, Michael;METCALF, Andrew, T.;MOHR, Peter, J.;MORENO, David, A.;NEWHOUSE, Brad;REN, Li;SCHWARZ, Jacob;CHEN, Huifen;GAZZARD, Lewis;SCHMIDT, Jane;DO, Steve WO2015/103137, 2015, A1 Location in patent:Paragraph 00178

5419-55-6
391 suppliers
$12.00/5g

22282-70-8
272 suppliers
$8.00/1g

401815-98-3
251 suppliers
$10.00/250mg

128071-98-7
293 suppliers
$7.00/1g

401815-98-3
251 suppliers
$10.00/250mg

688-74-4
332 suppliers
$10.40/25mL

22282-70-8
272 suppliers
$8.00/1g

401815-98-3
251 suppliers
$10.00/250mg