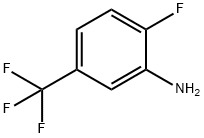
2-Fluoro-5-(trifluoromethyl)aniline synthesis
- Product Name:2-Fluoro-5-(trifluoromethyl)aniline
- CAS Number:535-52-4
- Molecular formula:C7H5F4N
- Molecular Weight:179.11

367-86-2
7439-89-6

535-52-4
General procedure for the synthesis of 2-fluoro-5-trifluoromethylaniline from 2-nitro-4-trifluoromethylfluorobenzene and iron powder: Referring to Example 1, 2-fluoro-5-trifluoromethylaniline was prepared as follows: 20 g of 3-nitro-4-fluorobenzotrifluoride was dissolved in 50 mL of methanol. Under stirring conditions, 16 g of iron powder was slowly added and concentrated hydrochloric acid was added dropwise. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, an appropriate amount of sodium bicarbonate was added to neutralize the reaction solution. Subsequently, ether was added for extraction and the insoluble material was removed by filtration using diatomaceous earth. The ether phase was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give 14 g of the target product 2-fluoro-5-trifluoromethylaniline in 82% yield.
Yield:535-52-4 82%
Reaction Conditions:
with hydrogenchloride;sodium hydrogencarbonate in methanol;diethyl ether;
Steps:
R.1 Production of 2-fluoro-5-trifluoromethylaniline
Reference Example 1 Production of 2-fluoro-5-trifluoromethylaniline Twenty grams of 3-nitro-4-fluorobenzotrifluoride was dissolved in 50 ml of methanol. An iron powder (16 g) was added and concentrated hydrochloric acid was added dropwise with stirring. The reaction mixture was stirred overnight, followed by addition of sodium bicarbonate for neutralization. Then diethyl ether was added, and the insolubles were filtered off with cerite. The ether phase was separated, dried and concentrated under reduced pressure, giving 14 g of the contemplated product (yield 82%).
References:
EP1243584,2002,A1

367-86-2
250 suppliers
$5.00/5g

535-52-4
270 suppliers
$5.00/1g
![Diazene, bis[3-(trifluoromethyl)phenyl]- (9CI)](/CAS/20180601/GIF/588-00-1.gif)
588-00-1
1 suppliers
inquiry

535-52-4
270 suppliers
$5.00/1g

402-44-8
290 suppliers
$9.00/1g

535-52-4
270 suppliers
$5.00/1g

121-17-5
26 suppliers
$19.00/250g

535-52-4
270 suppliers
$5.00/1g