
2-Fluoro-4-hydroxybenzonitrile synthesis
- Product Name:2-Fluoro-4-hydroxybenzonitrile
- CAS Number:82380-18-5
- Molecular formula:C7H4FNO
- Molecular Weight:137.11

105942-08-3

82380-18-5
General procedure for the synthesis of 2-fluoro-4-hydroxybenzonitrile from 4-bromo-2-fluorobenzonitrile: 4-bromo-2-fluorobenzonitrile (0.25 mmol), copper bromide (0.03 mmol), triethylamine (0.25 mmol, 1.0 eq.), formic acid (0.75 mmol, 3.0 eq.), and acetonitrile (179 mmol) were added to a reaction flask at room temperature. were used as reaction solvents. The reaction mixture was stirred at room temperature for 24 h under oxygen atmosphere. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, water (20 mL) and ethyl acetate (10 mL) were added to the reaction mixture for extraction, and the organic phases were combined and dried over anhydrous sodium sulfate. The dried organic phase was filtered and the filter cake was washed with ethyl acetate (5 mL × 3 times). Subsequently, the solvent was removed by rotary evaporator. The crude product was purified by column chromatography (eluent ratio: petroleum ether/ethyl acetate = 6:1) to afford the target product 2-fluoro-4-hydroxybenzonitrile in yellow liquid form in 82% yield.

105942-08-3
383 suppliers
$7.00/5g

82380-18-5
326 suppliers
$8.00/5g
Yield:82380-18-5 82%
Reaction Conditions:
with formic acid;oxygen;triethylamine;copper(ll) bromide in acetonitrile at 20; for 24 h;UV-irradiation;
Steps:
5 Example 5
4-Bromo-2-fluorobenzonitrile (0.25 mmol), copper bromide (0.03 mmol), triethylamine (0.25 mmol, 1.0 equiv), HCOOH (0.75 mmol, 3.0 equiv) at room temperature,179mmol of the reaction solvent CH3CN was added to the reaction tube, and the mixture was stirred at room temperature for 24 hours under the oxygen atmosphere.After the completion of the reaction was monitored by thin layer chromatography, 20 mL of water and 10 mL of ethyl acetate were added for extraction, followed by drying over anhydrous sodium sulfate, filtering after 5 minutes, washing the filter cake with ethyl acetate (5 mL×3 times), and then swirling off the solvent.The product was isolated by column chromatography (eluent: petroleum ether: ethyl acetate = 6:1), the product was a yellow liquid, yield 82%;
References:
CN107915586,2018,A Location in patent:Paragraph 0097-0100

3939-09-1
305 suppliers
$10.00/5g

82380-18-5
326 suppliers
$8.00/5g

94610-82-9
169 suppliers
$16.00/1g

82380-18-5
326 suppliers
$8.00/5g

348-27-6
208 suppliers
$15.00/1g

82380-18-5
326 suppliers
$8.00/5g
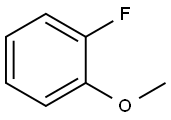
321-28-8
381 suppliers
$6.00/1g

82380-18-5
326 suppliers
$8.00/5g