
2-Fluoro-4-cyanobenzyl bromide synthesis
- Product Name:2-Fluoro-4-cyanobenzyl bromide
- CAS Number:105942-09-4
- Molecular formula:C8H5BrFN
- Molecular Weight:214.03
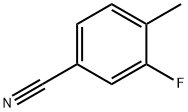
170572-49-3

105942-09-4
General procedure for the synthesis of 4-cyano-2-fluorobenzyl bromide from 3-fluoro-4-methylbenzonitrile: 3-fluoro-4-methylbenzonitrile (40 g, 296 mmol), N-bromosuccinimide (NBS, 63.2 g, 356 mmol), and benzoyl peroxide (3.6 g, 14.8 mmol) were dissolved in carbon tetrachloride (490 mL), and the reaction was performed at reflux for 16 hours. After completion of the reaction, the mixture was cooled to room temperature and filtered. The filtrate was concentrated and purified by fast column chromatography (eluent: 0-5% ethyl acetate/hexane) to afford the target product 4-(bromomethyl)-3-fluorobenzonitrile (35.4 g, 56% yield).

170572-49-3
274 suppliers
$7.00/5g

105942-09-4
216 suppliers
$8.00/1g
Yield:105942-09-4 94%
Reaction Conditions:
with NBS;2,2'-azobisisobutyronitrile in 1,2-dichloro-ethane at 75;Large scale;Reagent/catalyst;Temperature;Solvent;
Steps:
1; 2.1; 3.1; 4.1; 5.1 The first step:
550 g (1 eq) of 3-fluoro-4-methylbenzonitrile, 50 g (0.074 eq) of azobisisobutyronitrile and 1 kg of dichloroethane were added to the storage tank A. The solution was stirred, and 797 g (1.1 eq) of N-bromosuccinimide and 1.75 kg of dichloroethane were added to storage tank B. The microreactor 1 was heated to 75°C, and the solution in the storage tank A was sent to the first plate of the microreactor 1 through the pump A at a flow rate of 16 g/min; the solution in the storage tank B was passed through the slurry pump B at a flow rate of 25.5 g/min. It was sent to the first plate of microreactor 1; mixed reaction, after the bromination reaction of 6 plates, the color of the solution changed from light yellow to colorless, sampling GC detection, the reaction of raw materials was completed, product: dibromide = 98:2, The temperature was controlled at 20 to 35°C, and the reaction was dropped into 4 kg of water for quenching under stirring; post-processing: layering, the organic layer was washed once with 2 kg of water, The organic layer was concentrated to obtain 819.7 g of a light yellow solid product of 4-bromomethyl-3-fluorobenzonitrile, GC: 97.2%, and the isolated yield was 94%.
References:
CN113816874,2021,A Location in patent:Paragraph 0015; 0036-0041; 0043-0045; 0047-0049; 0051-0053

170572-49-3
274 suppliers
$7.00/5g

1146699-62-8
9 suppliers
inquiry

105942-09-4
216 suppliers
$8.00/1g

170572-49-3
274 suppliers
$7.00/5g

94-36-0
508 suppliers
$22.00/25g

105942-09-4
216 suppliers
$8.00/1g

219873-06-0
167 suppliers
$7.00/250mg

105942-09-4
216 suppliers
$8.00/1g
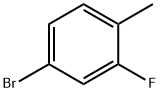
51436-99-8
344 suppliers
$5.00/1g

105942-09-4
216 suppliers
$8.00/1g