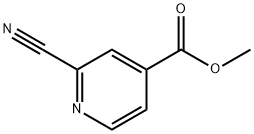
2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:2-CYANO-4-PYRIDINE CARBOXYLIC ACID METHYL ESTER
- CAS Number:94413-64-6
- Molecular formula:C8H6N2O2
- Molecular Weight:162.15
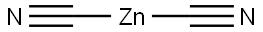
557-21-1
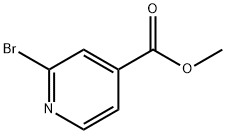
26156-48-9

94413-64-6
General procedure for the synthesis of methyl 2-cyano-4-pyridinecarboxylate from zinc cyanide and methyl 2-bromopyridine-4-carboxylate: methyl 2-bromopyridine-4-carboxylate (17.4 g, 80.5 mmol) was dissolved in N,N-dimethylformamide (DMF, 160 mL), and then zinc cyanide (Zn(CN)2, 5.7 g, 48.53 mmol) was added in a single step . The reaction mixture was degassed with nitrogen followed by the addition of tetrakis(triphenylphosphine)palladium (Pd(PPh3)4, 4.7 g). The mixture was again degassed and then the reaction was heated in an oil bath at 120 °C. After 2.5 hours of reaction, the reaction mixture was cooled to room temperature and water (200 mL) was added. The mixture was stirred for 30 minutes and subsequently filtered through a glass sand core funnel. The collected solid was washed with distilled water (2 x 100 mL) and then dried under vacuum to afford methyl 2-cyano-4-pyridinecarboxylate in 84% yield (11.0 g, 67.9 mmol). Electrospray mass spectrometry (ESI-MS) analysis showed [M + H]+ m/z 163.2 (C8H5N2O2 + H, calculated value: 162.1). The obtained product, methyl 2-cyano-4-pyridinecarboxylate, can be directly used in the subsequent reaction without further purification.
Yield:94413-64-6 94%
Reaction Conditions:
with copper(l) iodide;N,N-Dimethylcarbamoyl chloride in acetonitrile at 80; for 5 h;
Steps:
1 Example 1: Preparation of the compound of formula (4) Methyl 2-cyanoisonicotinate
The formula (5) compound methyl isobutyl niacin-N-oxide 1.53g (10mmol), b a carbamic chloride (0.11 ml, 11mmol), zinc cyanide (1.40g, 12mmol), cuprous iodide 0.19g (1mmol) dissolved in 50 ml of acetonitrile, mixtures in 80 °C reaction under 5 hours, TLC detection reaction (hexane/ethyl acetate = 1 the [...] 1), after the reaction, cooling to room temperature, add 20 ml water to stir reaction 5-30 minutes, removed the organic layer, the aqueous layer extracted with ethyl acetate three times, each 50 ml, combined with the organic layer, anhydrous sodium sulfate for drying, distilling solvent under reduced pressure, to obtain crude products, crude product using normal hexane/ethyl acetate = 4 the [...] 1 column chromatography, to obtain strawcoloured solid 1.53g, yield 94%, HPLC purity ( unitary method ): 97.7% ; melting point: 107.5-108.7 °C.
References:
CN104151297,2016,B Location in patent:Paragraph 0037; 0038
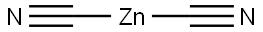
557-21-1
105 suppliers
$12.00/5g

26156-48-9
229 suppliers
$15.00/1g

94413-64-6
223 suppliers
$8.00/1g
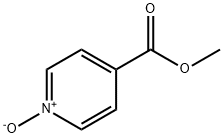
3783-38-8
87 suppliers
$11.00/1g

94413-64-6
223 suppliers
$8.00/1g

3783-38-8
87 suppliers
$11.00/1g

7677-24-9
393 suppliers
$19.00/5g
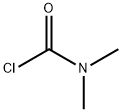
79-44-7
265 suppliers
$10.00/1g

94413-64-6
223 suppliers
$8.00/1g
