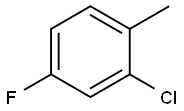
2-Chloro-4-fluorotoluene synthesis
- Product Name:2-Chloro-4-fluorotoluene
- CAS Number:452-73-3
- Molecular formula:C7H6ClF
- Molecular Weight:144.57
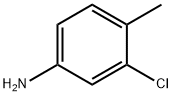
95-74-9

452-73-3
1. In a 1 L reactor (equipped with a condenser and a gas phase outlet diameter of 1/6 to 1/5 the diameter of the reactor), reduce the temperature to 20°C or lower. Slowly add 424 g (21.2 mol, 20 equiv.) of anhydrous hydrogen fluoride under stirring conditions, and further reduce the temperature after the addition is complete. 2. 150 g (1.059 mol, 1 eq.) of 3-chloro-4-methylaniline was added dropwise at 5 °C or lower, with the dropwise acceleration controlled to maintain the reaction temperature in the range of 5 to 15 °C, and the final addition was completed at about 3 °C. 3. after completion of the spiking, the temperature was lowered to 5 °C or lower, 73.1 g (1.06 mol, 1 eq.) of sodium nitrite was added in batches, and the reaction temperature was controlled to be in the range of -5 to 15 °C for 4 hours, followed by a heat hold for 1 hour. 4. Upon completion of the reaction, the reactor was programmed to warm up (strictly control the rate of warming: 0.5 to 1 °C per hour in the range of 0 to 20 °C; 1 to 2 °C per hour in the range of 20 to 80 °C), and was finally warmed up to 80 °C and held for 2 hours. 5. At the end of the holding time, reduce the temperature to 30 to 35°C and leave to stratify. The organic layer was neutralized to pH 7-8 with a dilute alkaline solution, followed by hydrodistillation. 6. 141 g of the organic layer at room temperature was collected and purity was 93.4% by gas chromatography. By distillation 125.1 g of the target product 2-chloro-4-fluorotoluene was obtained in 81.8% yield and 99.9% purity by gas chromatography.

95-74-9
250 suppliers
$9.00/25g

452-73-3
358 suppliers
$6.00/25g
Yield:452-73-3 81.8%
Reaction Conditions:
Stage #1:3-chloro-p-toluidine with hydrogen fluoride at 5 - 15; for 5 h;
Stage #2: with sodium nitrite at -5 - 80;Temperature;
Steps:
6 Example 6
1L reactor (equipped with a condenser, the gas phase outlet diameter is1/6 to 1/5 of thediameter of the kettle), and the temperature is lowered to 20 °C or lower. Under stirring, 424 g (21.2 mol, 20 eq) of anhydrous hydrogen fluoride is added, and the temperature is lowered after the feed is completed. To 5 °C or less,150 g (1.059 mol, 1 eq) of 3-chloro-4-methylaniline wasadded dropwise, and the temperature was controlled in the range of 5 to 15 °C, and the addition was completed in about 3 hours, and the temperature was kept for 2 hours.After thecompletion of theheat preservation, thetemperature was lowered to 5 °C or less, and 73.1 g of sodium nitrite (1.06 mol, 1 eq) was added in portions, and the reaction temperature was controlled within the rangeof-5 to 15 °C, and the mixture was added forabout 4 hours, and the heat was kept for 1 hour.After the heat preservation, the temperature of the reaction kettle is programmed (strictly controlling the heating rate:when thetemperature is in the range of 0 to 20 °C, the temperature is raised by 0.5 to 1 °C per hour,when the temperature is in the range of 20 to 80 °C, the temperature is raised by 1 to 2 °C per hour), and the temperature is raised to 80 °C and keep warm for2h.After the heat preservation is completed, the temperature is lowered to 30 to 35 °C, and the layers are separated. The organic layer is neutralized to a pH of 7 to 8 by a dilute alkali, and steam distillation is carried out. The organic layer is 141 g at room temperature, and the purity of the gas chromatographic detection is 93.4%. The distillation obtained 125.1 g of qualified product, the yield was 81.8%, and the purity of 2-chloro-4-fluorotoluene obtained by gas chromatography was 99.9%.
References:
Liaoning Tian Yu Chemical Co., Ltd.;Jiangsu Lianhua Technology Co., Ltd.;Lianhua Science And Technology (Yancheng) Co., Ltd.;Lianhua Technology Co., Ltd.;Zhao Liqiang;Yang Zongsheng;Yuan Weiwei;Yin Xiaoxu;Du Yong;Yang Wenlong;Wang Yang;Li Shanshan CN108569948, 2018, A Location in patent:Paragraph 0035-0059