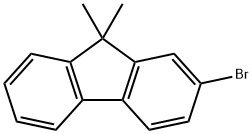
2-Bromo-9,9-dimethylfluorene synthesis
- Product Name:2-Bromo-9,9-dimethylfluorene
- CAS Number:28320-31-2
- Molecular formula:C15H13Br
- Molecular Weight:273.17
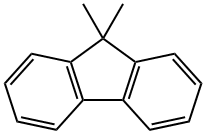
4569-45-3

28320-31-2
General procedure for the synthesis of 9,9-dimethyl-2-bromofluorene from 9,9-dimethylfluorene: 1) Add 5 g of 9,9-dimethylfluorene, 0.35 g of benzyltriethylammonium bromide, and 0.77 g of sodium bromate to a 100 mL round bottom flask; 2) In another 100 mL round-bottomed flask fitted with a thermometer and a constant-pressure dropping funnel, 34.5 mL of water and 23 mL of methylene chloride were added through the constant-pressure dropping funnel; 3) Stir the liquid in the mixed flask at atmospheric pressure until the solid is completely dissolved, controlling the reaction temperature to 10 °C; 4) Slowly add 7.5 mL of 40% by weight hydrobromic acid dropwise to the reaction mixture of step 3 via a constant pressure dropping funnel, and after completion of the dropwise addition, continue to stir the reaction for 1.5 hours at room temperature; 5) The reaction solution from step 4 was allowed to stand and layered to separate the aqueous phase from the organic phase; 6) Perform one dichloromethane extraction of the aqueous phase, the extract is combined with the organic phase of step 5, and the combined organic phase is washed three times with distilled water; 7) The organic phase of step 6 was dried with anhydrous sodium sulfate, filtered and concentrated, and the resulting product was recrystallized with ethanol and dried under vacuum at 60 °C. The final yield of 9,9-dimethyl-2-bromofluorene was obtained as 73%, and the HPLC detection showed that the purity of the product was 90%, and the product was white solid powder.
Yield:28320-31-2 98%
Reaction Conditions:
Stage #1: 2-Bromofluorenewith benzyl-triethyl-ammonium chloride;sodium hydroxide in lithium hydroxide monohydrate;dimethyl sulfoxide at 20; for 0.333333 h;Inert atmosphere;
Stage #2: iodomethane in lithium hydroxide monohydrate;dimethyl sulfoxide; for 4 h;Inert atmosphere;
Steps:
1.S1 S1: make the compound of formula (1) undergo methylation reaction to generate the compound of formula (2):
This step is specifically: at room temperature, in a 200mL single-neck round-bottom flask,Add 1 g of the compound of formula (1) and 20 mL of dimethyl sulfoxide,Then 2 ml of 50% aqueous sodium hydroxide solution and 50 mg of TEBAC were added to the system,After stirring for 20 min, 0.9 g of methyl iodide was added, and stirring was continued for 4 h;Quenched with water, extracted with dichloromethane, dried over anhydrous magnesium sulfate, and distilled under reduced pressure to remove the solvent;The crude product was purified by column chromatography (eluent: petroleum ether) to obtain 1.1 g of the compound of formula (2) as a white solid with a yield of 98%.
References:
CN114249746,2022,A Location in patent:Paragraph 0039; 0043-0045

4569-45-3
219 suppliers
$7.00/5g

28320-31-2
314 suppliers
$5.00/1g

