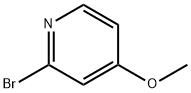
2-Bromo-4-methoxypyridine synthesis
- Product Name:2-Bromo-4-methoxypyridine
- CAS Number:89488-29-9
- Molecular formula:C6H6BrNO
- Molecular Weight:188.02
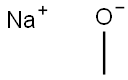
124-41-4
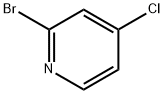
22918-01-0

89488-29-9
The general procedure for the synthesis of 2-bromo-4-methoxypyridine from sodium methanol and 2-bromo-4-chloropyridine was as follows: sodium methanol (0.35 g, 6.48 mmol) was added to a solution of 2-bromo-4-chloropyridine (1.0 g, 5.2 mmol) in dimethyl sulfoxide (DMSO, 10 mL). After the addition was completed, the reaction mixture was stirred at 120 °C for 24 hours. After completion of the reaction, the mixture was cooled to room temperature and extracted with ethyl acetate (EtOAc). The organic layers were combined and dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure. Purification of the residue by preparative high performance liquid chromatography (HPLC) gave 2-bromo-4-methoxypyridine (0.25 g, 25% yield) as a colorless oil. The mass spectrometry (MS) electrospray ionization (ESI) calculated value was 189 for C6H6BrNO [M + H]+ and the measured value was 189.

620-08-6
316 suppliers
$5.00/1g

89488-29-9
153 suppliers
$12.00/250mg
Yield: 62%
Reaction Conditions:
Stage #1:4-methoxypyridine with n-butyllithium;2-(N,N-dimethylamino)ethanol in hexane at -20; for 1 h;Inert atmosphere;
Stage #2: with 1,2-dibromo-1,1,2,2-tetrachloroethane in tetrahydrofuran;hexane at -78 - 20;Inert atmosphere;
Steps:
4-Methoxy-2-bromopyridine (2).
A solution of N,N-dimethylethanolamine (1.40 mL, 13.93 mmol) in hexanes (10 mL) at -20 oC was treated with n-BuLi (11.91 mL, 27.86 mmol, 2.34M in hexanes). The reaction mixture was stirred under nitrogen for 30 minutes. Neat 4-methoxypyridine (0.70 mL, 6.90 mmol) was added dropwise. The orange solution was stirred for one hour and then cooled to -78 oC. A solution of 1,2-dibromo-1,1,2,2-tetrachloroethane (5.40 g, 16.57 mmol) in THF (5 mL) was added dropwise, and the mixture was allowed to slowly warm to room temperature overnight. The reaction was quenched with water at 0 oC and extracted with diethyl ether. The combined extracts were dried over anhydrous magnesium sulfate and concentrated. The desired product was distilled (70 °C, 3 mmHg) to yield 0.81 g (62%) of 2 as yellow oil. 1H NMR (300 MHz, CDCl3) δ 3.86 (s, 3H), 6.78-6.80 (dd, J 2.1, 2.4, 5.9 Hz, 1H), 7.00-7.01 (d, J 2.4 Hz, 1H), 8.16-8.18 (d, J 6.0 Hz, 1H);13C NMR (75 MHz, CDCl3) δ 55.6, 110.2, 113.2, 143.0, 150.6, 166.8; FTIR (film) 2321, 2350, 2371, 2851, 2925,2956 cm-1; HRMS (FAB) calcd for C6H6BrNO (M+1) 187.9711; found 187.9708.
References:
Bori, Ibrahim D.;Comins, Daniel L. [Arkivoc,2021,vol. 2021,p. 57 - 72]
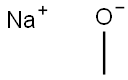
124-41-4
727 suppliers
$12.00/25g

22918-01-0
264 suppliers
$6.00/1g

89488-29-9
153 suppliers
$12.00/250mg

89488-33-5
1 suppliers
inquiry

89488-29-9
153 suppliers
$12.00/250mg

36953-40-9
148 suppliers
$25.00/1g

74-88-4
358 suppliers
$15.00/10g

89488-29-9
153 suppliers
$12.00/250mg
109-04-6
540 suppliers
$6.00/25g

89488-29-9
153 suppliers
$12.00/250mg