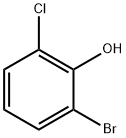
2-Bromo-4-chlorophenol synthesis
- Product Name:2-Bromo-4-chlorophenol
- CAS Number:695-96-5
- Molecular formula:C6H4BrClO
- Molecular Weight:207.45

17005-59-3

695-96-5
The general procedure for the synthesis of 2-bromo-4-chlorophenol from the compound (CAS:17005-59-3) is as follows: in a 10 mL round-bottomed flask with a magnetic stir bar, phenols or olefins (0.1 mmol), carbon tetrabromide (CBr4, 33 mg, 0.1 mmol), anhydrous acetonitrile (CH3CN, 1 mL), and dichloro tris(2,2'-) bipyridine)ruthenium(II) (Ru(bpy)3Cl2, 3.8 mg, 0.005 mmol). The reaction mixture was irradiated at room temperature using a blue LED lamp (1 W) and air was continuously passed until the complete disappearance of the raw material was shown by thin layer chromatography (TLC). After completion of the reaction, the solvent was removed by distillation under reduced pressure. Finally, the residue was purified by fast column chromatography to obtain the target product 2-bromo-4-chlorophenol.
Yield:695-96-5 86%
Reaction Conditions:
Stage #1:2-hydroxybromobenzene with sulfuric acid in acetonitrile at 20; for 0.0833333 h;
Stage #2: with N-chloro-succinimide in acetonitrile at 20; for 3 h;regioselective reaction;Reagent/catalyst;
Steps:
Representative procedure for the preparation of compound 11a
General procedure: To a solution of phenol (470.6 mg, 5 mmol) in MeCN (10 mL) was added con. H2SO4 (285 μL, 1.05 equiv.) at room temperature, the mixture was stirred for 5 min. Then, NBS (934.4 mg, 1.05 equiv.) was added to the mixture. The reaction was monitored using TLC analysis. The mixture was evaporated to dryness. H2O (15 mL) was added to the residue and extracted with CH2Cl2 (3 x 15 mL). The combined organic layers were washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure to afford the crude product. The major product was isolated using silica gel chromatography to obtain the product as light yellow liquid; yield: 79% (0.69 g).
References:
Wu, Yong-Qi;Lu, Hai-Jia;Zhao, Wen-Ting;Zhao, Hong-Yi;Lin, Zi-Yun;Zhang, Dong-Feng;Huang, Hai-Hong [Synthetic Communications,2020,vol. 50,# 6,p. 813 - 822] Location in patent:supporting information
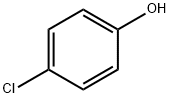
106-48-9
498 suppliers
$10.00/5 g

695-96-5
269 suppliers
$7.00/5g

17005-59-3
1 suppliers
inquiry

695-96-5
269 suppliers
$7.00/5g

5324-13-0
86 suppliers
$100.00/1g

695-96-5
269 suppliers
$7.00/5g