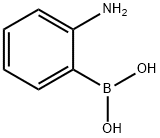
2-Aminophenylboronic acid synthesis
- Product Name:2-Aminophenylboronic acid
- CAS Number:5570-18-3
- Molecular formula:C6H8BNO2
- Molecular Weight:136.94

5570-19-4

5570-18-3
General procedure for the synthesis of 2-aminophenylboronic acid from 2-nitrophenylboronic acid: (i) Preparation of 2-aminophenylboronic acid: 2-nitrophenylboronic acid (500 mg, 2.99 mmol) was dissolved in methanol (10 mL) and 10% Pd/C (250 mg) was added. The reaction mixture was stirred at atmospheric pressure for 2 h under hydrogen atmosphere. Upon completion of the reaction, the catalyst was removed by diatomaceous earth filtration and the filtrate was concentrated under reduced pressure. The residue was purified by column chromatography (silica gel, dichloromethane solution of 0-50% CMA) to afford the target compound 2-aminophenylboronic acid (182 mg).1H NMR (300 MHz, CD3OD) δ 6.69-7.75 (m, 4H).

615-43-0
425 suppliers
$5.00/5g

7732-18-5
499 suppliers
$12.69/100ml

73183-34-3
587 suppliers
$6.00/5g

5570-18-3
203 suppliers
$8.00/100mg
Yield:5570-18-3 78.1%
Reaction Conditions:
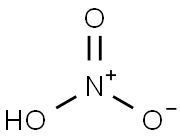
Stage #1:2-iodophenylamine;bis(pinacol)diborane with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in dimethyl sulfoxide at 80; for 18 h;Inert atmosphere;
Stage #2:water with sodium periodate;ammonium chloride in methanol at 20; for 18 h;
Steps:
4.6 preparation of 2-aminophenyl boronic acid
The 1.10g to iodine aniline (5mmol), 1.40g connecting boric acid pinacone ester (5.5mmol), 1.45g acetic acid potassium (15mmol) and PdCl2(dppf) catalyst (150 mg) by adding 50 ml in two neck bottles, vacuumizing and nitrogen-filling 6 times, the state of the protection of nitrogen by adding DMSO (25 ml), 80 °C reaction 18h, in a water cooling retrodisplacement, precipitate after filtering, washing, drying to obtain the crude product purification column chromatography (petroleum ether: ethyl acetate = 5:1) shall be near white powder solid 0.99g, yield: 90.0%. The 0.88g the above-mentioned preparation of the compound (4mmol) by adding 100 ml round-bottom flask, add 30 ml of methanol is dissolved, add 0.56gNH4Cl (10mmol), constant voltage dropping funnel for slowly dripping 2.56gNaIO4(12mmol) of 7 ml aqueous solution, stirring the mixture at room temperature for reaction 18h, TLC detection, filtering to remove the inorganic salt, methanol filters after evaporation to dryness, drying to obtain the crude product purification column chromatography (petroleum ether: ethyl acetate = 1:5) shall be near white powdery solid 0.43g, yield 78.1%.
References:
Ocean University of China;Jiang, Tao;Yin, ruijuan;Zhang, Li Juan CN103497211, 2016, B Location in patent:Paragraph 0183; 0184

5570-19-4
319 suppliers
$8.00/1g

5570-18-3
203 suppliers
$8.00/100mg

98-80-6
743 suppliers
$5.00/5g

5570-18-3
203 suppliers
$8.00/100mg
191171-55-8
239 suppliers
$7.00/250mg

5570-18-3
203 suppliers
$8.00/100mg