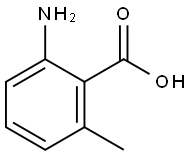
2-Amino-6-methylbenzoic acid synthesis
- Product Name:2-Amino-6-methylbenzoic acid
- CAS Number:4389-50-8
- Molecular formula:C8H9NO2
- Molecular Weight:151.16

13506-76-8

4389-50-8
General procedure for the synthesis of 2-amino-6-methylbenzoic acid from 2-methyl-6-nitrobenzoic acid: Pd/C catalyst (150 mg) was added to a methanol (35 mL) solution of 2-methyl-6-nitrobenzoic acid (1.5 g, 8.29 mmol). The reaction mixture was stirred for 2 hours at room temperature under hydrogen atmosphere. Upon completion of the reaction, the mixture was filtered to remove the catalyst and the filtrate was subsequently concentrated to give 2-amino-6-methylbenzoic acid (1.2 g) in yellow solid form. The product was characterized by liquid chromatography-mass spectrometry (LCMS, ESI mode): the calculated value of C8H9NO2 [M+H]+ was 152 and the measured value was 152, as expected.

13506-76-8
260 suppliers
$6.00/1g

4389-50-8
274 suppliers
$6.00/1g
Yield:4389-50-8 100%
Reaction Conditions:
palladium
Steps:
R.17 2-Amino-6-methylbenzoic acid
REFERENCE EXAMPLE 17 2-Amino-6-methylbenzoic acid To a methanolic solution (150 ml) of 6-methyl-2-nitrobenzoic acid (14.25 g) was added palladium-on-carbon (1.40 g) and the hydrogenation reaction was conducted at atmospheric temperature and pressure (hydrogen consumption 5.3 1). The catalyst was filtered off and the filtrate was concentrated under reduced pressure to provide the title compound as light-yellow solid (15.12 g; yield 100%). 1 H-NMR (CDCl3) δ: 2.47(3H,s), 6.50(2H,t,J=8.1Hz), 7.00-7.07(4H,m). IR (KBr): 2927, 2645, 1645, 1599, 1545, 1470, 1394, 1334, 1288, 1236, 813, 775, 580, 419 cm-1.
References:
Takeda Chemical Industries, Ltd. US5753664, 1998, A

40637-78-3
15 suppliers
inquiry

4389-50-8
274 suppliers
$6.00/1g
1885-76-3
98 suppliers
$43.50/1g

4389-50-8
274 suppliers
$6.00/1g

13506-76-8
260 suppliers
$6.00/1g

4389-50-8
274 suppliers
$6.00/1g