
2-Amino-6-bromopyridine synthesis
- Product Name:2-Amino-6-bromopyridine
- CAS Number:19798-81-3
- Molecular formula:C5H5BrN2
- Molecular Weight:173.01
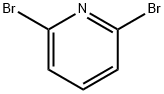
626-05-1
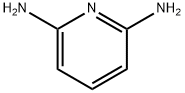
141-86-6

19798-81-3
14.1 Preparation of 6-bromo-2-aminopyridine In a steel autoclave equipped with a glass liner, 10.00 g of 2,6-dibromopyridine (42.2 mmol) was suspended in 50 mL of concentrated ammonia. The autoclave was sealed and heated to 190°C using a heating jacket and maintained for 6 hours (pressure was raised to about 25 bar). After the autoclave was cooled and depressurized, the reaction mixture was mixed with 100 mL of ethyl acetate for phase separation. The aqueous phase was extracted twice with ethyl acetate, 100 mL each. the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was dissolved in 250 mL of a solvent mixture of cyclohexane/ethyl acetate (1:1, v/v) to remove the by-product 2,6-diaminopyridine. Subsequently, the solution was filtered through a short silica gel column (5 x 20 cm) and washed again with 250 mL of cyclohexane/ethyl acetate (1:1, v/v) mixed solvent. After removal of the solvent under reduced pressure, the residue was sublimated at 90°C and 10-1 mbar to give 6.49 g (37.5 mmol, 88.9% yield) of 6-bromo-2-aminopyridine as a white solid.
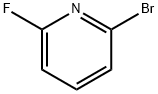
144100-07-2
241 suppliers
$10.00/5g

19798-81-3
451 suppliers
$5.00/1g
Yield:19798-81-3 93%
Reaction Conditions:
with pentanamidine hydrochloride;sodium t-butanolate in diethylene glycol dimethyl ether;water at 150; for 24 h;
Steps:
7 preparation of 6-bromo-2-amino-pyridine
2-fluoro-6-bromo-pyridine (1 mmol) was added to a 25 ml reaction tube.Pentamidine hydrochloride (2mmol), sodium tert-butoxide (3mmol),H2O (0.5 mL) and diethylene glycol dimethyl ether (2.5 mL).The reaction is carried out at 150 ° C for a reaction time of 24 hours.Cool to room temperature.The reaction was quenched by adding 10 mL of ethyl acetate, and washed with 6 mL of saturated brine.The organic phase was separated, and the aqueous phase was extracted three times with ethyl acetate (ethyl acetate (6 mL)).The solvent is distilled off under reduced pressure, including an organic solvent and an inorganic solvent, the organic solvent,The title product was isolated by column chromatography, yield 93%.
References:
CN109232402,2019,A Location in patent:Paragraph 0061; 0062

626-05-1
511 suppliers
$9.00/10g

19798-81-3
451 suppliers
$5.00/1g
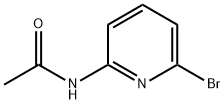
25218-99-9
55 suppliers
$20.00/250mg

19798-81-3
451 suppliers
$5.00/1g

626-05-1
511 suppliers
$9.00/10g
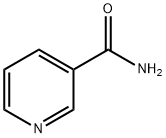
98-92-0
1253 suppliers
$5.00/5g

19798-81-3
451 suppliers
$5.00/1g
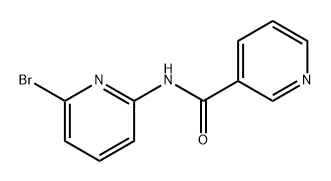
1140964-62-0
0 suppliers
inquiry