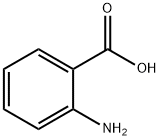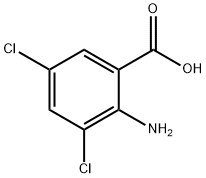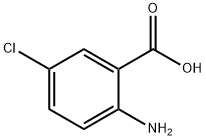
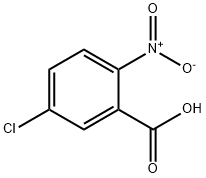
2-Amino-5-chlorobenzoic acid synthesis
- Product Name:2-Amino-5-chlorobenzoic acid
- CAS Number:635-21-2
- Molecular formula:C7H6ClNO2
- Molecular Weight:171.58
2516-95-2

635-21-2
General procedure for the synthesis of 2-amino-5-chlorobenzoic acid from 5-chloro-2-nitrobenzoic acid: active Raney nickel (2 g) was added to the reaction flask, followed by the addition of an ethanol solution of 5-chloro-2-nitrobenzoic acid (20 g, 110 mmol). The reaction mixture was stirred at room temperature in a hydrogen atmosphere overnight. Upon completion of the reaction, the reaction solution was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure to give 16 g of white solid product (96% yield). The product was characterized by 1H NMR (DMSO-d6): δ 6.77 (d, J = 8.9 Hz, 1H), 7.24 (dd, J = 2.9, 8.9 Hz, 1H), 7.62 (d, J = 2.9 Hz, 1H), 8.7 (br, 3H); EIMS: 170 (M-H).

2516-95-2
324 suppliers
$5.00/5g

635-21-2
426 suppliers
$5.00/5g
Yield:635-21-2 96%
Reaction Conditions:
with hydrogen;nickel in ethanol at 20;
Steps:
3 Preparation of 2-Amino-5-chloro benzoic acid (Compound 57)
To a solution of 5-chloro-2-nitrobenzoic acid (20 g, 110 mmol) in ethanol was added freshly activated raney nickel (2 g). The mixture was stirred overnight at room temperature under hydrogen atmosphere. The solution was filtered through celite and evaporated under reduced pressure to yield 16 g (96%) of white SOLIDS. 1H NMR (DMSO-D6) : d 6.77 (d, J = 8.9 Hz, 1H), 7.24 (DD, J= 2.9, 8.9 Hz, 1H), 7.62 (d, J= 2.9 Hz, 1H), 8.7 (b, 3H); EIMS: 170 (M-H).
References:
AVANIR PHARMACEUTICALS WO2004/74218, 2004, A2 Location in patent:Page 115-116

88279-11-2
6 suppliers
inquiry

635-21-2
426 suppliers
$5.00/5g

17422-32-1
381 suppliers
$6.00/1g

635-21-2
426 suppliers
$5.00/5g
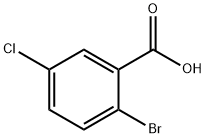
21739-93-5
256 suppliers
$5.00/1g

635-21-2
426 suppliers
$5.00/5g