
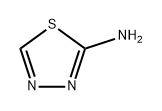
2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE synthesis
- Product Name:2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE
- CAS Number:37566-39-5
- Molecular formula:C2H2BrN3S
- Molecular Weight:180.03
4005-51-0
![2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE](/CAS/GIF/37566-39-5.gif)
37566-39-5
General procedure for the synthesis of 2-amino-5-bromo-1,3,4-thiadiazole from 2-amino-1,3,4-thiadiazole: 1. A solution of 2-amino-1,3,4-thiadiazole (5 g, 48.45 mmol) in methanol (70 mL) was added to a reaction flask. 2. Sodium bicarbonate (8.14 g, 96.90 mmol) and bromine (2.5 mL, 48.45 mmol) were added sequentially. 3. The reaction mixture was stirred at room temperature until the complete disappearance of the ingredients was demonstrated by thin layer chromatography (TLC) monitoring (about 30-40 minutes). 4. After completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. 5. The crude product was ground with a solvent mixture of methanol-ether (1:1, v/v) to give 2-amino-5-bromo-[1,3,4]thiadiazole as a white solid. 6. The mother liquor containing a small amount of product was purified by fast column chromatography using dichloromethane-methanol (99:1, v/v) as eluent. 7. The product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 300 MHz, DMSO-d6): δ 7.52 (br s, 2H). 8. Mass spectrometry (ES+) analysis showed m/z 181 ([M+H]+, calculated value: 180.0). Yield: 99%.
64-18-6
1127 suppliers
$22.12/250ML

79-19-6
511 suppliers
$14.00/1g
![2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE](/CAS/GIF/37566-39-5.gif)
37566-39-5
218 suppliers
$24.00/10g
Yield:37566-39-5 85%
Reaction Conditions:
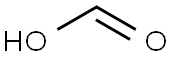
Stage #1:formic acid;thiosemicarbazide with sulfuric acid in water for 3 h;Reflux;
Stage #2: with ammonium hydroxide;bromine in water; pH=3.5 - 8
Stage #3: in water
Steps:
5-bromo-l, 3, 4-thiadiazol-2-amine VI1 mol of V was suspended in 50 ml of 85% water formic acid and 80 ml of sulfuric acid were carefully added. Reaction mixture was stirred for 3 hours on boiling water bath and then cooled down. 800 ml of water were added and reaction mixture was alkalified by water ammonia solution to ph 3.5. Then reaction mixture was heated to 550C and 68.5 ml of Br2 were added dropwise under liquid layer, keeping on temperature 550C and ph 3.5. Reaction mixture was stirred at this temperature overnight and after that it was cooled down and basified by water ammonia solution to ph 8. Precipitate which was formed was filtered off, washed by water and dried to provide title compound 153.02 g (85%).
References:
URIFER LTD.;NIR, Uri;SHPUNGIN, Sally;YAFFE, Etai;COHEN, Moshe WO2010/97798, 2010, A1 Location in patent:Page/Page column 17

4005-51-0
294 suppliers
$5.00/10g
![2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE](/CAS/GIF/37566-39-5.gif)
37566-39-5
218 suppliers
$24.00/10g