
2-AMINO-4-BROMOBENZYL ALCOHOL synthesis
- Product Name:2-AMINO-4-BROMOBENZYL ALCOHOL
- CAS Number:946122-05-0
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05

135484-83-2

946122-05-0
Step 1: Methyl 2-amino-4-bromobenzoate (500 mg, 2.17 mmol) was dissolved in anhydrous THF (10 mL) and the solution was cooled to -10 °C. Under stirring, lithium aluminum hydride (1 M solution of THF, 6.5 mL, 6.52 mmol) was slowly added dropwise. After the dropwise addition was completed, the reaction mixture was allowed to gradually warm up to room temperature and stirred at room temperature overnight. Upon completion of the reaction, the mixture was cooled in an ice-water bath and the reaction was quenched by sequential addition of methanol and saturated potassium sodium tartrate solution. The mixture was continued to be stirred at room temperature for 6 hours. Subsequently, the reaction mixture was diluted with a mixture of ethyl acetate and water to separate the organic and aqueous phases. The organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness under reduced pressure to give (2-amino-4-bromophenyl)methanol (Ex.44a, 326 mg, 74% yield) as a viscous brown solid.
22996-19-6
127 suppliers
inquiry

946122-05-0
71 suppliers
$24.00/250mg
Yield:946122-05-0 90%
Reaction Conditions:
with iron;ammonium chloride in ethanol;water at 75; for 1 h;
Steps:
73.2
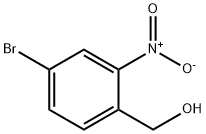
Step 2. A mixture of (4-bromo-2-nitro-phenyl)-methanol (1.85 g, 7.97 mmol), iron powder (2.23 g, 39.9 mmol), ammonium chloride (213 mg, 3.99 mmol), ethanol (20 mL), and water (10 mL) was heated at 75° C. for 1 h, then after cooling filtered through a pad of diatomaceous earth. The filtrate was evaporated and the residue partitioned between ethyl acetate and water, the organic layer was washed with brine, dried (MgSO4), and evaporated to produce (2-amino-4-bromo-phenyl)-methanol (1.53 g, 90%). Off-white solid, MS (EI)=201.0 (M+).
References:
Ackermann, Jean;Bleicher, Konrad;Ceccarelli, Simona M.;Chomienne, Odile;Mattei, Patrizio;Sander, Ulrike Obst US2007/191603, 2007, A1 Location in patent:Page/Page column 40

135484-83-2
182 suppliers
$6.00/1g

946122-05-0
71 suppliers
$24.00/250mg

20776-50-5
320 suppliers
$10.00/1g

946122-05-0
71 suppliers
$24.00/250mg