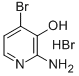
2-AMINO-3-HYDROXY-4-BROMOPYRIDINE HBR synthesis
- Product Name:2-AMINO-3-HYDROXY-4-BROMOPYRIDINE HBR
- CAS Number:114414-17-4
- Molecular formula:C5H6Br2N2O
- Molecular Weight:269.9219
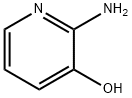
16867-03-1

114414-17-4
General procedure for the synthesis of 2-amino-4-bromopyridin-3-ol hydrobromide from 2-amino-3-hydroxypyridine: Bromine (11.2 mL, 0.21 mol) was slowly added dropwise to a mechanically stirred suspension of 2-amino-3-hydroxypyridine (20 g, 0.18 mol) in acetic acid (300 mL) at 5-10 °C, after which the reaction system was brought to room temperature. The reaction mixture was heated to 120-125 °C, maintained for 12 h and then concentrated to give the crude product. The crude product was ground with ether (50 mL x 3) and subsequently dried under vacuum to afford 2-amino-4-bromopyridin-3-ol hydrobromide as a dark brown solid, which could be used in subsequent reactions without further purification. Yield: 48.5 g; MS (m/z): 189.3, 191.1 ([M + H]+).

16867-03-1
589 suppliers
$10.00/25g

114414-17-4
71 suppliers
$130.00/250mg
Yield: 74%
Reaction Conditions:
with bromine in ethanol at 0 - 20; for 72 h;
Steps:
Step 1 . Synthesis of compound (242)
To a solution of 2-amino-2-hydroxypyridine 241 (10.Og, 91 mmol) in absolute ethanol (50mL) at 0°C was dropwise added bromine (5.2ml, 102mmol) and the mixture was stirred at room temperature for 3 days. The solvent was removed under reduced pressure and low temperature. The residue was cooled to 0°C. AcOEt (1 00mL) was added and the mixture was stirred for 1 h. The suspension was filtered off (washing with AcOEt). The red-brown solid recovered was dried under reduced pressure leading to the expected product 242 (Yield: 74%, 18.2g).
References:
ANAGENESIS BIOTECHNOLOGIES S.A.S. WO2021/13712, 2021, A1 Location in patent:Page/Page column 154