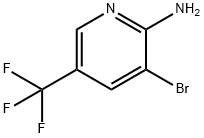
2-Amino-3-bromo-5-(trifluoromethyl)-pyridine synthesis
- Product Name:2-Amino-3-bromo-5-(trifluoromethyl)-pyridine
- CAS Number:79456-30-7
- Molecular formula:C6H4BrF3N2
- Molecular Weight:241.01
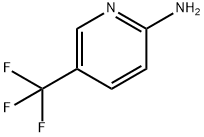
74784-70-6

79456-30-7
The general procedure for the synthesis of 2-amino-3-bromo-5-trifluoromethylpyridine from 2-amino-5-trifluoromethylpyridine was as follows: 71 g of N-bromosuccinimide was added in batches to a mixture containing 65 g of 2-amino-5-trifluoromethylpyridine and 100 ml of chloroform under cooling conditions in an ice-water bath, with the temperature increasing during the reaction. Subsequently, the reaction mixture was stirred at room temperature for 1 hour. Next, the reaction mixture was heated to 80°C and stirred at this temperature for 30 minutes. Upon completion of the reaction, the mixture was cooled to room temperature and quenched by sequentially adding saturated aqueous sodium thiosulfate and saturated aqueous sodium bicarbonate, followed by extraction with chloroform. The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. Finally, the residue was purified by silica gel column chromatography to give 96 g of the target product 2-amino-3-bromo-5-trifluoromethylpyridine. The structure of the product was confirmed by 1H-NMR (CDCl3): δ 8.27 (1H, d), 7.86 (1H, d), 5.38 (2H, brs).

74784-70-6
330 suppliers
$5.00/1g

79456-30-7
133 suppliers
$6.00/250mg
Yield:79456-30-7 94.17%
Reaction Conditions:
with N-Bromosuccinimide in tetrahydrofuran at 0 - 20; for 1 h;Temperature;Solvent;
Steps:
57; 63 3-Bromo-5-(trifluoromethyl)pyridin-2-amine
To a solution of 5- (trifluoromethyl)pyridin-2-amine (10 g, 61.69 mmol) in THF (100 mL) was added NBS (11.53 g, 64.77 mmol) portionwise at 0 °C and the mixture was stirred at 20 °C for 1 h. The mixture was concentrated under vacuum and purified by silica gel column chromatography to give 3-bromo- 5-(trifhioromethyl)pyridin-2-amine (14 g, 58.09 mmol, 94.17% yield).
References:
WO2020/219871,2020,A1 Location in patent:Paragraph 00499; 00529

79456-29-4
6 suppliers
inquiry

74784-70-6
330 suppliers
$5.00/1g

79456-30-7
133 suppliers
$6.00/250mg