
2,6-DIAMINO-4-METHYL PYRIDINE synthesis
- Product Name:2,6-DIAMINO-4-METHYL PYRIDINE
- CAS Number:38439-33-7
- Molecular formula:C6H9N3
- Molecular Weight:123.16

108-89-4

38439-33-7
General procedure for the synthesis of 2,6-diamino-4-methylpyridine from 4-methylpyridine: General method: synthesis of 4-methylpyridine-2,6-diamine (1). Method A: 4-Methylpyridine (15 g, 161.1 mmol) was suspended in mineral oil (220 mL), sodium amide (27.0 g, 483.3 mmol, 3 eq.) was added and stirred overnight at room temperature. Subsequently, the homogeneous suspension was heated to 130 °C and maintained for 15 min, and the beginning of hydrogen precipitation was observed. The temperature was gradually increased to 160 °C over 30 min, the color of the suspension changed from light brown to dark brown and the mixture formed a foamy solid. Continuing heating to 200 °C over 30 min, hydrogen release accelerated and the color of the mixture changed to black. After 3 hours of reaction at 215 °C, complete consumption of the feedstock and completion of hydrogen release was confirmed by TLC (unfolding agent: ethyl acetate/methanol/acetic acid, 10:1:0.1) monitoring. The reaction mixture was cooled to room temperature, the black solid was collected by filtration and washed with petroleum ether (5 x 100 mL). Silica gel (100 g) and 96% ethanol (130 mL) were carefully added to the black solid and evaporated to dryness after 20 min. Subsequently, the solid was placed on top of a suction filter containing silica gel (300 g; ethyl acetate) and eluted using a gradient of ethyl acetate to ethyl acetate/isopropanol (8:1), the product grade was collected and evaporated. The brown residue was recrystallized with tert-butyl methyl ether/diisopropyl ether to give 13.1 g of target product 1 as gray crystals in 66% yield. Method B: The compound was prepared by a similar method to 1 using 2-amino-4-methylpyridine (15 g, 138.7 mmol) and sodium amide (16.2 g, 416.1 mmol, 3 equiv) as starting materials. Yield: 13.2 g (76%). Melting point: 100-102 °C. 1H NMR (DMSO-d6) δ: 5.46 (s, 2H, pyridine ring H), 5.21 (s, 4H, NH2), 1.95 (s, 3H, CH3). 13C{1H} NMR (DMSO-d6) δ: 158.7 (pyridine ring C), 148.1 (pyridine ring C), 96.2 (pyridine ring C). 20.7 (CH3).HRMS (ESI):[M + H]+, measured value 124.0869.Theoretically calculated value 124.0875 for C6H9N3.
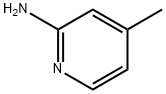
695-34-1
546 suppliers
$5.00/10g

38439-33-7
85 suppliers
$76.00/250mg
Yield:38439-33-7 67%
Reaction Conditions:
with tetralin;sodium amide at 200; for 4 h;Inert atmosphere;
References:
Trapani, Mariachiara;Castriciano, Maria Angela;Collini, Elisabetta;Bella, Giovanni;Cordaro, Massimiliano [Organic and Biomolecular Chemistry,2021,vol. 19,# 37,p. 8118 - 8127]

108-89-4
345 suppliers
$14.00/25mL

38439-33-7
85 suppliers
$76.00/250mg
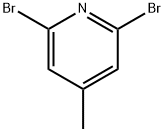
73112-16-0
131 suppliers
$15.00/250mg

38439-33-7
85 suppliers
$76.00/250mg
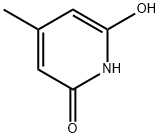
4664-16-8
87 suppliers
$54.89/250mg

38439-33-7
85 suppliers
$76.00/250mg