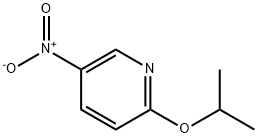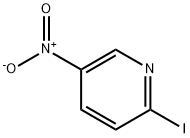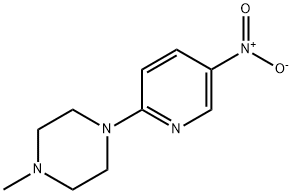
2-(4-Methylpiperazin-1-yl)-5-nitropyridine synthesis
- Product Name:2-(4-Methylpiperazin-1-yl)-5-nitropyridine
- CAS Number:55403-34-4
- Molecular formula:C10H14N4O2
- Molecular Weight:222.24
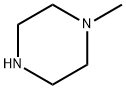
109-01-3

4548-45-2

55403-34-4
General procedure for the synthesis of 5-nitro-2-(4-methyl-1-piperazinyl)pyridine from N-methylpiperazine and 2-chloro-5-nitropyridine: 2-chloro-5-nitropyridine (3.16 g, 20.0 mmol), N-methylpiperazine (2.00 g, 20.0 mmol) and potassium carbonate (2.76 g, 20.0 mmol) were reacted in a N,N- Dimethylformamide (DMF) in a stirred mixture was heated to 100 °C and reacted for 24 hours. After completion of the reaction, the mixture was cooled to room temperature, poured into water and extracted with dichloromethane (CH2Cl2). The organic phases were combined, dried with anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure. The obtained residue was purified by fast column chromatography (silica gel as stationary phase, eluent was a 10:90 ethanol:ethyl acetate mixture containing 2% ammonia) to afford 5-nitro-2-(4-methyl-1-piperazinyl)pyridine as an off-white solid in the yield of 4.0 g (90% yield), and the structure of the product was confirmed by nuclear magnetic resonance (NMR) analysis.
Yield:55403-34-4 99%
Reaction Conditions:
in acetonitrile; for 1.5 h;Heating / reflux;
Steps:
4
Intermediate 4l-Methyl-4-(5-nitro-2-pyridinyl)piperazineTo a solution of 2-bromo-5-nitropyridine (22.3 g, 110 mmol) in CH3CN (200 mL) was added JV-methylpiperazine (30.5 mL, 275 mmol) and the resulting mixture was heated with stirring to reflux. After 90 min, the reaction was cooled to room temperature and concentrated to dryness. The solids were partitioned between water and EtOAc. The organic layer was separated and washed with brine, dried (MgSO4), filtered and concentrated to dryness affording the title compound as a yellow solid (24.2 g, 99%). LC-MS (ES) m/z = 223 [M+H]+.
References:
WO2008/147831,2008,A1 Location in patent:Page/Page column 17