
2,4-Dichloro-5-thiazolecarboxaldehyde synthesis
- Product Name:2,4-Dichloro-5-thiazolecarboxaldehyde
- CAS Number:92972-48-0
- Molecular formula:C4HCl2NOS
- Molecular Weight:182.03

2295-31-0
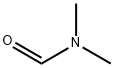
68-12-2

92972-48-0
General procedure for the synthesis of 2,4-dichlorothiazole-5-carboxaldehyde (330): 2,4-thiazolidinedione (329, 2.7 g, 23.07 mmol) was dissolved in N,N-dimethylformamide (DMF, 1.23 mL, 15.98 mmol) at 0 °C and protected by argon and slowly added dropwise to phosphorus trichloride (8.15 mL, 87.17 mmol) (8.15 mL, 87.17 mmol), and the dropwise addition time was controlled at 15 min. Subsequently, the reaction mixture was gradually warmed to room temperature and stirred continuously for 1 hour. Next, the reaction system was heated to 120 °C and stirring was continued for 4 hours. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was carefully poured into pre-cooled ice water and extracted with dichloromethane (CH2Cl2, 3 x 100 mL). The organic phases were combined, washed sequentially with saturated sodium bicarbonate solution (100 mL) and water (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by silica gel column chromatography with 5% ethyl acetate/hexane as eluent, and the target compound 330 (1.4 g, 33% yield) was finally obtained as a brown oil.TLC conditions: 30% ethyl acetate/hexane (Rf=0.8).1H NMR (500 MHz, DMSO-d6): δ9.87 (s, 1H).

2295-31-0
523 suppliers
$6.00/5g

92972-48-0
184 suppliers
$21.00/1g
Yield:-
Reaction Conditions:
with trichlorophosphate in N,N-dimethyl-formamide
Steps:
20 Scheme 12--Route to Preferred Group 13 B & E Nitrogen
Scheme 20--Route to Preferred Group 44 [4',5':2,3] [4,5-d] Isomer Reaction of thiazolidin-2,4-dione with POCl3 and DMF gives 2,4-dichlorothiazole-5-carbaldehyde.
References:
Warner-Lambert Company US5679683, 1997, A