
2,4-DICHLORO-3-AMINOPYRIDINE synthesis
- Product Name:2,4-DICHLORO-3-AMINOPYRIDINE
- CAS Number:173772-63-9
- Molecular formula:C5H4Cl2N2
- Molecular Weight:163

5975-12-2

173772-63-9
2,4-Dichloro-3-nitropyridine (2.058 g, 10 mmol) was dissolved in acetic acid (10 mL) under nitrogen protection. Iron powder (1.9191 g, 34.4 mmol) was subsequently added. The reaction mixture was heated and stirred at 40 °C for 2 hours. After completion of the reaction, the mixture was poured into ice water and adjusted to neutral with sodium bicarbonate. The reaction mixture was extracted with ethyl acetate (3 x 100 mL). The combined organic phases were washed once with saturated sodium bicarbonate solution (1 x 100 mL). The aqueous layer was back-extracted once more with ethyl acetate (100 mL). All organic phases were combined, dried with magnesium sulfate, filtered and concentrated under reduced pressure to give 3-amino-2,4-dichloropyridine (1.510 g, 9.26 mmol, 87% yield).

5975-12-2
233 suppliers
$10.00/1g

173772-63-9
131 suppliers
$11.00/250mg
Yield: 1.2 g (90%)
Reaction Conditions:
in HCl (conc.);diethyl ether;water
Steps:
37.c EXAMPLE 37
(c) 3-Amino-2,4-dichloropyridine. 2,4-Dichloro-3-nitropyridine (1.5 g, 8 mmol) was dissolved in Et2O (8 mL). A solution of SnCl2.2H2O (18 g, 80 mmol) in HCl (conc.) (18 mL) was added cautiously. The reaction was exothermic upon this addition and the Et2O boiled off of the solution. The reaction mixture was allowed to stir overnight at room temperature. The solution was cooled to 0° C. in an ice-water bath and the precipitate was collected via filtration. The resulting solid was suspended in distilled H2O, and the mixture was adjusted to neutral pH by the addition of concentrated NH4OH at 0° C. The resulting solution was extracted with EtOAc (2*). The combined extracts were washed with brine, dried over MgSO4, filtered and concentrated in vacuo to afford 1.2 g (90%) of the title compound as a colorless crystalline solid. 1H NMR (DMSO-d6; 500 MHz): δ 5.88 (s,2) 7.35 (d, 1, J=5.1), 7.63 (d, 1, J=5.1). MS m/z: 163 (M+1).
References:
Amgen Inc. US6187777, 2001, B1

5975-12-2
233 suppliers
$10.00/1g

173772-63-9
131 suppliers
$11.00/250mg

626-03-9
349 suppliers
$5.00/1g

173772-63-9
131 suppliers
$11.00/250mg

89282-12-2
256 suppliers
$8.00/5g

173772-63-9
131 suppliers
$11.00/250mg

5975-12-2
233 suppliers
$10.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
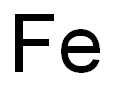
7439-89-6
462 suppliers
$10.00/25g

173772-63-9
131 suppliers
$11.00/250mg