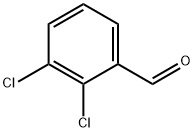
2,3-Dichlorobenzaldehyde synthesis
- Product Name:2,3-Dichlorobenzaldehyde
- CAS Number:6334-18-5
- Molecular formula:C7H4Cl2O
- Molecular Weight:175.01
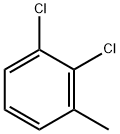
32768-54-0

6334-18-5
(1) Device construction: Configure the tubular reactor system with reference to Figure 2, using a (3a + 3e) type DC channel combined with tubing of Corning Heart Cel 1 construction. Select the appropriate pipe diameter and reactor volume according to the reactant flow rate and required reaction time. Heat transfer oil was selected as the heat transfer medium. (2) Solution preparation: The catalyst solution was prepared by dissolving 6.06 g of cobalt acetate and 6.06 g of sodium molybdate in 200 mL of a mixed solvent of 2,3-dichlorotoluene and 200 mL of acetic acid, where the molar ratio of cobalt acetate to 2,3-dichlorotoluene was 0.015:1. The hydrogen peroxide-acetic acid solution was also prepared by dissolving 6.06 g of sodium bromide in a 25% aqueous hydrogen peroxide solution. The molar ratio of sodium bromide to 2,3-dichlorotoluene was 0.015:1. (3) Reaction process: When the molar ratio of hydrogen peroxide to 2,3-dichlorotoluene was 2:1, 2,3-dichlorotoluene-acetic acid solution and hydrogen peroxide-acetic acid solution were pumped into a preheated microchannel reactor (shown in Fig. 2) at flow rates of 5.56 mL/min and 111 mL/min, respectively. The reaction temperature was controlled to be 90 °C and the residence time was 1200 s. After the reaction was completed, the outlet material was cooled to 0 °C and the reaction was quenched with methylene chloride. The conversion of 2,3-dichlorotoluene was 42.8% and the yield of 2,3-dichlorobenzaldehyde was 29.0% as analyzed by gas chromatography.

38594-42-2
109 suppliers
$12.00/250mg

6334-18-5
425 suppliers
$6.00/10g
Yield:6334-18-5 48.19 g
Reaction Conditions:
with hydrogen bromide;dihydrogen peroxide in 1,4-dioxane at 25; for 7 h;Temperature;
Steps:
1
In a 1L reactor,add 61.5 g of 2,3-dichlorotoluene, 3.075 g of azobisisobutyronitrile (5%), 215.25 g of 2-dichloroethane, heat the mixture at 75 ° C and add 44 g of bromine drop wise,reflux reaction till red bromine fades , Intermittent drip and 136.2g27.5% hydrogen peroxide continues to react till red bromine no longer generates. Add a 49.2g30% aqueous solution of sodium carbonate to the reaction solution, reflux reaction for 8 h ,at the same time recover1,2-dichloroethane.The hydrolyzate is heated and filtered at 80° C, into organic phase add 246g 1,4-Dioxane , add 40% of the catalyst HBr6.15g, add 27.5% hydrogen peroxide 38.3g,react at 25 ° C for 7 h. The reaction system is extracted three times with 1,2-dichloroethane,the organic phase is concentrated and obtained crude 2,3-dichlorobenzaldehyde, re-crystallization from ethanol and obtained 48.19 g of 2,3-dichlorobenzaldehyde, GC purity is 99.26% and yield is 72.1%.
References:
Lianyungang Industry Investment Group Co., Ltd.;Jiang Honglai;Jiang Xiao;Han Yong;Zang Han;Zhu Chengjie;Shen Weiwei CN107032968, 2017, A Location in patent:Paragraph 0015; 0017; 0018; 0019; 0022

32768-54-0
266 suppliers
$9.00/25g

6334-18-5
425 suppliers
$6.00/10g

4414-54-4
40 suppliers
$43.00/1g

6334-18-5
425 suppliers
$6.00/10g
288-43-7
1 suppliers
inquiry
1820-80-0
481 suppliers
$5.00/5g

6287-38-3
264 suppliers
$8.00/10g

6334-18-5
425 suppliers
$6.00/10g
95-50-1
597 suppliers
$10.00/5g

6334-18-5
425 suppliers
$6.00/10g