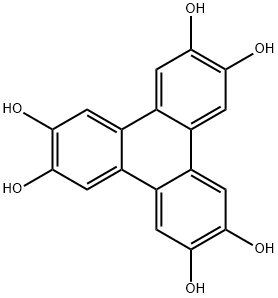
2,3,6,7,10,11-Triphenylenehexol synthesis
- Product Name:2,3,6,7,10,11-Triphenylenehexol
- CAS Number:4877-80-9
- Molecular formula:C18H12O6
- Molecular Weight:324.28
808-57-1

4877-80-9
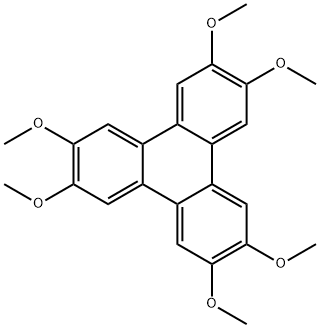
The general procedure for the synthesis of 2,3,6,7,10,11-hexamethoxytriphenylene from 2,3,6,7,10,11-hexahydroxytriphenylene was carried out as follows: the preparation of compound 4 was carried out with reference to the method described in literature [14]. Hexamethoxytriphenylene (7 g, 17.14 mmol) was dissolved in dichloromethane (50 ml) and the resulting solution was cooled in an ice bath. Subsequently, boron tribromide solution (11.47 ml) was slowly added dropwise to the reaction mixture over a period of 30 min. After completion of the dropwise addition, the reaction mixture was stirred at room temperature overnight. After completion of the reaction, the mixture was slowly poured into crushed ice (100 g) and stirred vigorously until the ice was completely melted. Next, multiple extractions were carried out with ethyl acetate (6 x 150 ml), the organic phases were combined and dried over anhydrous sodium sulfate, and finally concentrated to dryness under reduced pressure to give a dark purple solid product. The yield of this step was nearly quantitative and the resulting compound could be used in subsequent reactions without further purification.

120-80-9
532 suppliers
$13.00/25g

4877-80-9
148 suppliers
$49.00/250mg
Yield:4877-80-9 83.1%
Reaction Conditions:
with ammonium persulfate;sulfuric acid in water at 20; for 7 h;Product distribution / selectivity;
Steps:
1
34.2 g of ammonium persulfate (0.15 mol) was added to 16.5 g of catechol (0.15 mol) dispersed in 50 ml of a 70 wt% aqueous solution of sulfuric acid. The mixture was stirred for 7 hours at room temperature, and the resultant precipitate was then filtered and washed with water. 300 ml of acetone and 1.5 g of activated carbon were added to the precipitate, the mixture was stirred for 30 min at room temperature, and insoluble matter was subsequently filtered off from the mixture. 300 ml of ion- exchanged water was added to the filtrate, and then acetone was distilled off at distillation temperatures of 56 to 100°C under normal pressure (101.3 kPa). The resultant precipitate was filtered and dried under reduced pressure to afford 14.2 g of crystals of HHTP (yield: 83.1%; purity > 99%).
References:
OTSUKA CHEMICAL COMPANY, LIMITED EP1676826, 2006, A1 Location in patent:Page/Page column 4

808-57-1
90 suppliers
$17.00/250mg

4877-80-9
148 suppliers
$49.00/250mg
91-16-7
589 suppliers
$13.00/25g

4877-80-9
148 suppliers
$49.00/250mg