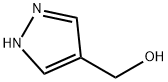
(1H-PYRAZOL-4-YL)METHANOL synthesis
- Product Name:(1H-PYRAZOL-4-YL)METHANOL
- CAS Number:25222-43-9
- Molecular formula:C4H6N2O
- Molecular Weight:98.1
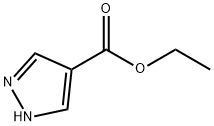
37622-90-5

25222-43-9
In a flame-dried round-bottom flask (RBF), a suspension of lithium aluminum hydride (LAH, 45.2 mL, 45.2 mmol, 1 M in tetrahydrofuran (THF)) was cooled to 0°C. Subsequently, a solution of ethyl 1H-pyrazole-4-carboxylate (3.17 g, 22.6 mmol) dissolved in THF (20 mL) was added slowly and dropwise. The reaction mixture was gradually warmed to room temperature and stirred overnight at room temperature. Upon completion of the reaction, the mixture was cooled in an ice bath and 1.36 mL of water and 10 mL of 1 M sodium hydroxide (NaOH) solution were added sequentially dropwise to carefully quench the reaction, followed by stirring for 20 min. Solid magnesium sulfate (MgSO4) was added to dry the reaction mixture, and stirring was continued at room temperature for 30 min after removal of the ice bath. The solids were removed by filtration through diatomaceous earth (CELITE) and the filter cake was washed with THF and methanol (MeOH), respectively. The filtrates were combined and evaporated to give the target product (1H-pyrazol-4-yl) methanol (1.75 g, 78.9% yield) as a white solid. The product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 500 MHz, DMSO-d6): δ 12.58 (broad single peak, 1H), 7.58 (single peak, 1H), 7.40 (single peak, 1H), 4.74 (triple peak, J = 5.5 Hz, 1H), 4.37 (double peak, J = 5.2 Hz, 2H).
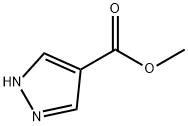
51105-90-9
114 suppliers
$9.00/100mg

25222-43-9
113 suppliers
$12.00/100mg
Yield: 75.78%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 4 h;
Steps:
19.2 Step-2: Preparation of (lH-Pyrazo-4-yI)-methanol:
To the stirred solution of methyl 1H- pyrazole-4-carboxylate 19-2 ( 19 g, 150.66 mmol) in THF (500 mL) was added lithium aluminium hydriede (8.58 g, 225.99 mmol) portionwise at 0°C. The reaction mixture was stirred at room temperature for 4 hours and then cooled to room temperature and quenched by Fieser workup. The organic later was filtered through celite and concentrated under reduced pressure to afford 1H- pyrazol-4-ylmethanol 19-3 ( 1 1.2 g, 114.17 mmol, 75.78% yield) as white solid. LC MS: ES+ 99 0
References:
C4 THERAPEUTICS, INC.;VEITS, Gesine, Kerstin;HE, Minsheng;HENDERSON, James, A.;NASVESCHUK, Christopher, G.;PHILLIPS, Andrew, J.;GOOD, Andrew, Charles WO2019/191112, 2019, A1 Location in patent:Page/Page column 318

37622-90-5
340 suppliers
$6.00/1g

25222-43-9
113 suppliers
$12.00/100mg

70817-17-3
14 suppliers
inquiry

25222-43-9
113 suppliers
$12.00/100mg